A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient and must refer to the package insert, product label and/or instructions for use before using any Stryker product.
Shown to improve CPR quality, on the move and over long durations5-8,16
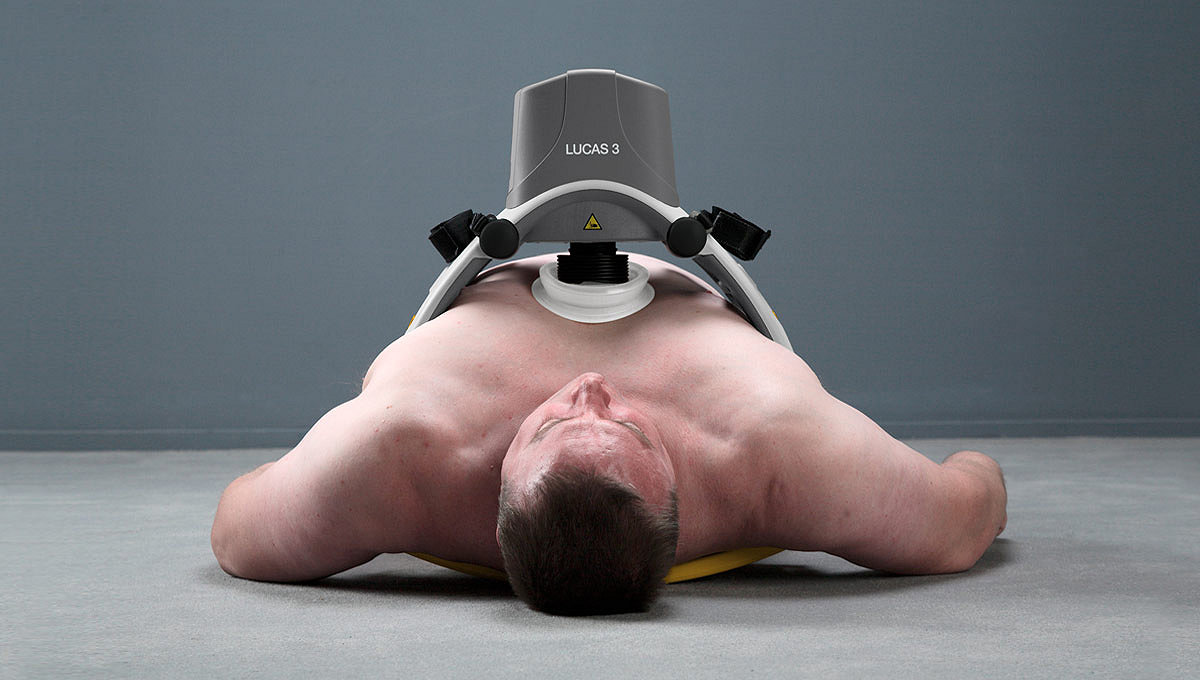
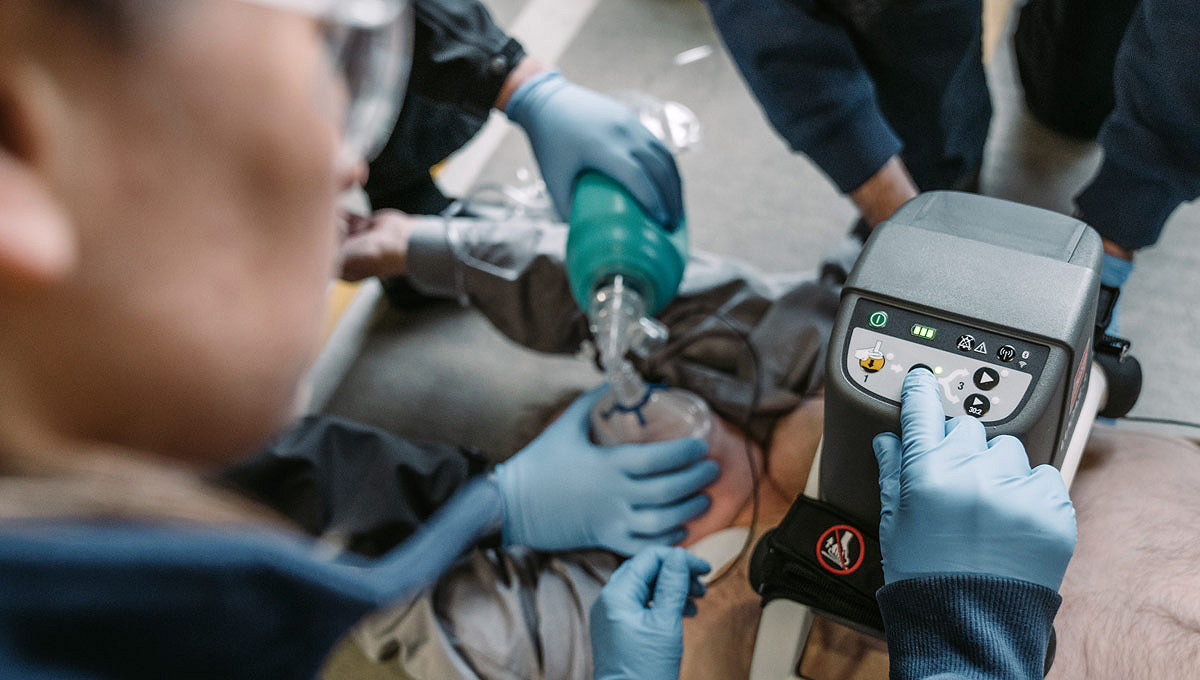
The LUCAS device extends the reach of care by maintaining chest compressions during transport to advanced lifesaving therapies, including ECMO (extracorporeal membrane oxygenation) or PCI (percutaneous coronary intervention) in the cath lab. By increasing provider safety37, avoiding fatigue over long durations38 and reducing transport risks by allowing caregivers to sit belted2,39, the LUCAS device can help calm the scene and provide an extra pair of hands.
50,000+
With more than 50,000 devices in the global market, a patient is treated approximately every minute9,10
>99%
Operational reliability in clinical use11
+60%
Increased blood flow to the brain vs. manual CPR12

Improve CPR quality
By using automated, guidelines-consistent CPR, the LUCAS device provides consistent and high-quality chest compressions shown by research to increase the chances of good patient outcomes13. In studies, it was shown that the LUCAS device has been demonstrated to increase blood flow to the brain12,14 and achieve higher EtCO2 values compared to manual compressions.15
High-quality CPR with fewer interruptions16
Delivery of high-quality CPR is vital for patient outcomes. The LUCAS device delivers chest compressions with guidelines-consistent depth of 5.3 cm / 2.1 inches and rate of 102 per minute1.*
* LUCAS 3, version 3.1 can be configured for depth and rate.
Extend your care reach
Delivers consistent, high-quality chest compressions for as long as the patient requires them.17 * Serves as a bridge to care for patients that have not responded to traditional resuscitation interventions. The LUCAS device provides perfusion during angiography and PCI in the cath lab.18
Keep your team safe
Help reduce caregiver risk during patient transport and in the cath lab with potentially reduced x-ray exposure and less body strain to the CPR provider.
Enhance team efficiency
Focus on other life-saving tasks like diagnosis and the treatment of underlying conditions.6
* When using multiple batteries or an external power source, battery typically lasts for 45 minutes of operation.20
Product highlights
LUCAS web training
Read, listen and watch how to apply the LUCAS chest compression system for training and re-training purposes.
Users are encouraged to do refresher training at least once a year. Please select your LUCAS device below.

FAQs
The following questions and answers apply only to the LUCAS 3, v3.1.
Assembled dimensions: 22.0 x 20.5 x 9.4 inches20
In carrying case dimensions: 22.8 x 13.0 x 10.2 inches20
The rate of compressions is 102 +/- two compressions per minute20
The device with battery (no straps) weighs 17.7 pounds. The battery weighs 1.3 pounds.20
There is no patient weight limit. The chest height can be between 6.7 to 11.9 inches with a maximum chest width of 17.7 inches.20
Interested in learning more? Connect with an expert.
M0000014366 REV AA
Manufacturer
Jolife AB, a part of Stryker
Scheelevägen 17
Ideon Science Park
SE-223 70 Lund, Sweden
Tel: +46 (0) 46 286 50 00
info@lucas-cpr.com

ProCare Services
Our excellent field service team stands beside you to make sure your lifesaving devices are ready when you need them. Highly experienced, our field service team knows to use, care for, maintain and repair your device better than anyone, giving your devices the longest possible life.
Technical support
We'll work with you to quickly assess your situation and find the best solution.
1. Beesems SG, Hardig BM, Nilsson A, Koster RW, Force and depth of mechanical chest compressions and their relation to chest height and gender in an out-of-hospital setting, Resuscitation, 2015;91:67-72.
2. Becker L, Zaloshnja E, Levick N, et al. Relative risk of injury and death in ambulances and other emergency vehicles. Accident analysis and prevention. 2003;35(6): 941-948.
3. Jones A, Lee R. Cardiopulmonary resuscitation and back injury in ambulance officers. International Archives of Occupational and Environmental Health.2005 May;78 (4); 332-336.
4. Edelson, et al. Interim guidance for basic and advanced life support in adults, children, and neonates with suspected or confirmed COVID-19. Circulation. 2020.
5. Putzer G, Braun P, Zimmerman A, et al. LUCAS compared to manual cardiopulmonary resuscitation is more effective during helicopter rescue–a prospective, randomized, cross-over manikin study. Am J Emerg Med.2013 Feb;31(2):384-9.
6. Gyory R, Buchle S, Rodgers D, et al. The efficacy of LUCAS in prehospital cardiac arrest scenarios: A crossover mannequin study. West J Emerg Med.2017;18(3):437-45.
7. Magliocca A, Olivari D, De Giorgio D, et al. LUCAS Versus Manual Chest Compression During Ambulance Transport: A Hemodynamic Study in a Porcine Model of Cardiac Arrest. Journal of the American Heart Association 2018;8(1).
8. Steen S, Liao Q, Pierre L, et al. Evaluation of LUCAS, a new device for automatic mechanical compression and active decompression resuscitation. Resuscitation. 2002;3(55): 285-299.
9. Data on file, Stryker, Lindroth S, sales volume over 8 years, 2025.
10. Data on file, Stryker Lindroth S, frequency calculation.
11. Rubertsson S, Lindgren E, Smekal D, et al. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in out-of-hospital cardiac arrest. The LINC randomized trial. JAMA. 2013;311(1):53-61.
12. Carmona Jiménez F, Padró PP, García AS, et al. Cerebral flow improvement during CPR with LUCAS, measured by Doppler. Resuscitation. 2011, 82S1:30, AP090. [Also published in a longer version, in Spanish with English abstract, in Emergencias. 2012;24:47-49].
13. Sporer K, Jacobs M, Derevin L, et al. Continuous quality improvement efforts increase survival with favorable neurologic outcome after out-of-hospital cardiac arrest. Prehosp Emerg Care. 2016;14:1-6.
14. Rubertsson S, Karlsten R. Increased cortical cerebral blood flow with LUCAS; a new device for mechanical chest compressions compared to standard external compressions during experimental cardiopulmonary resuscitation. Resuscitation 2005:65(3);357-363.
15. Axelsson C, Karlsson T, Axelsson A, et al. Mechanical active compression-decompression cardiopulmonary resuscitation (ACD-CPR) versus manual CPR according to pressure of end tidal carbon dioxide (PETCO2) during CPR in out-of-hospital cardiac arrest (OHCA). Resuscitation. 2009, 80(10):1099-1103.
16. Olasveengen TM, Wik L, Steen PA. Quality of cardiopulmonary resuscitation before and during transport in out-of-hospital cardiac arrest. Resuscitation. 2008;76(2):185-90.
17. Forti A, Brugnaro P, Rauch S, et al. Hypothermic Cardiac Arrest With Full Neurologic Recovery After Approximately Nine Hours of Cardiopulmonary Resuscitation: Management and Possible Complications. Ann Emerg Med. 2019;73(1):52-57.
18. William P, Rao P, Kanakadandi U, et al. Mechanical cardiopulmonary resuscitation in and on the way to the cardiac catheterization laboratory. Circ J. 2016:25;80(6):1292-1299.
19. Pocock H, Deakin C, Quinn T, et al. Human factors in pre-hospital research: lessons from the PARAMEDIC trial. Emerg Med J Online. Feb 25, 2016.
20. Data on file, Stryker LUCAS 3 chest compression system Instructions for Use 101034-00.
21. Morrison L, Gent L, Lang E, et al. Part 2: Evidence Evaluation and Management of Conflicts of Interest 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132[suppl 2]:S368–S382.
22. Koster R W, Beenen L F, Van der Boom E B, et al. Safety of mechanical chest compression devices AutoPulse and LUCAS in cardiac arrest: a randomized clinical trial for non-inferiority. European Heart Journal. 2017;38:3006-3013.
23. Saussy JM., et al. Optimization of Cardiopulmonary Resuscitation With an Impedance Threshold Device, Automated Compression Cardiopulmonary Resuscitation and Post-Resuscitation in-the-Field Hypothermia Improves Short-term Outcomes Following Cardiac Arrest. Circulation. 23 November 2010; 122: A256.
24. Maule Y. Mechanical external chest compression: A new adjuvant technology in cardiopulmonary Resuscitation. (Translated from French language: L’assistance cardiaque externe: nouvelle approche dans la RCP.) Urgences & Accueil. 2007;29:4-7
25. Pepe PE., et al. Abstract 15255: How Would Use of Flow-Focused Adjuncts, Passive Ventilation and Head-Up CPR Affect All-Rhythm Cardiac Arrest Resuscitation Rates in a Large, Complex EMS System? Circulation. 2016;134:A15255
26. Anantharaman V., et al. Prompt use of mechanical cardiopulmonary resuscitation in out-of-hospital cardiac arrest: the MECCA study report. Singapore Med J. 2017; 58(7): 424-31.
27. Venturini JM., et al. Mechanical chest compressions improve rate of return of spontaneous circulation and allow for initiation of percutaneous circulatory support during cardiac arrest in the cardiac catheterization laboratory. Resuscitation. 2017 Jun;115:56-60.
28. Rolston, DM, Li T, Owens C, et al. Mechanical, team-focused, video-reviewed cardiopulmonary resuscitation improves return of spontaneous circulation after emergency department implementation. J Am Heart Assoc. 2020;9:e014420.
29. Crowley CP, Wan ES, Salciccioli JD, et al. The Use of Mechanical Cardiopulmonary Resuscitation May Be Associated With Improved Outcomes Over Manual Cardiopulmonary Resuscitation During Inhospital Cardiac Arrests. Crit Care Explor. 2020 Nov; 2(11):e0261.
30. Wagner H., et al. Mechanical chest compressions in the coronary catheterization laboratory to facilitate coronary intervention and survival in patients requiring prolonged resuscitation efforts. Scand J Trauma Resusc Emerg Med. 2016; 24:4.
31. Esibov A., et al. Mechanical chest compressions improved aspects of CPR in the LINC trial. Resuscitation. 2015 Jun;91:116-21.
32. Kamrud J, Boland L, Frazee C, et al. Use of transthoracic impedance data to evaluate intra-arrest chest compression quality. International Journal of Critical Care and Emergency Medicine. (2016) 2:014.
33. Tranberg T., et al. Quality of cardiopulmonary resuscitation in out-of-hospital cardiac arrest before and after introduction of a mechanical chest compression device, LUCAS-2; a prospective, observational study. Scandinavian Journal of Trauma Resuscitation and Emergency Medicine. (2015) 23:37.
34. Yannopoulos D, Bartos J, Martin C, et al. Minnesota Resuscitation Consortium’s Advanced Perfusion and Reperfusion Cardiac Life Support Strategy for Out-of-Hospital Refractory Ventricular Fibrillation. Journal of the American Heart Association. 2016;5:e003732, originally published June 13, 2016.
35. Bonnemeier H, Simonis G, Olivecrona G, et al. Continuous mechanical chest compression during in-hospital cardiopulmonary resuscitation of patients with pulseless electrical activity. Resuscitation. 2011;82:155-9
36. Wagner H, Terkelsen C, Friberg H, et al. Cardiac arrest in the catheterisation laboratory: A 5-year experience of using mechanical chest compressions to facilitate PCI during prolonged resuscitation efforts. Resuscitation. 2010;81(4):383-387.
37. AHA Guidelines: Panchal A, Bartos J, Cabanas J, et al. Part 3: Adult Basic and Advanced Life Support. 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2), S366–S468.
38. Sugerman NT, Edelson DP, Leary M, et al. Rescuer fatigue during actual inhospital cardiopulmonary resuscitation with audiovisual feedback: a prospective multicenter study. Resuscitation 2009;80:981-4.
39. Fox J, Fiechter R, Gerstl P, et al. Mechanical versus manual chest compression CPR under ground ambulance transport conditions. Acute Cardiac Care 2013;15:1-6.
40. Libungan B, Dworeck C, Omerovic E. Successful percutaneous coronary intervention during cardiac arrest with use of an automated chest compression device: A case report. Therapeutics and Clinical Risk Management. 2014;10:255-257.
41. Maule Yves. Assistance cardiaque externe : Masser mieux, mais surtout masser plus. URGENCE PRATIQUE. 2011 No106. (article in French)
42. Levy M, Yost D, Walker R, et al., A quality improvement initiative to optimize use of a mechanical chest compression device within a high-performance CPR approach to out-of-hospital cardiac arrest resuscitation. Resuscitation. 2015;92:32-37
EC-LUC-SYK-1570318_REV-1_en_us