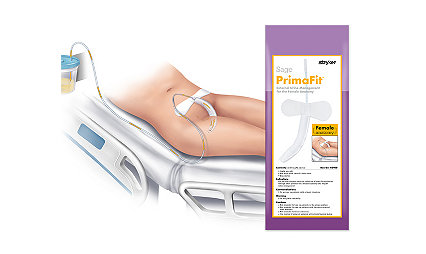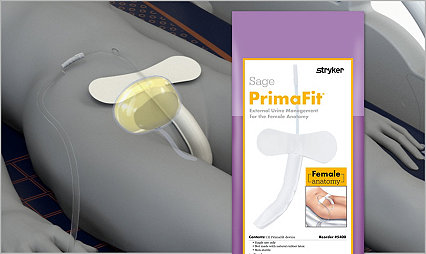
Your patients need a better solution
Catheter-associated urinary tract infections (CAUTIs) pose a threat to patient safety, often leading to complications like sepsis, prolonged hospital stays and increased healthcare costs.1 Traditional indwelling catheters can cause discomfort, skin injury and infection. Many other external female catheter devices are universally designed, aren’t approved for use on adolescents and most need to be replaced several times a day.
No Catheter. No Cauti.TM
It's time for a change
The Sage PrimaFit External Urine Management for the Female Anatomy is a great fit for your patients and your hospital. It’s designed to help improve efficiency and reduce costs as well as address the #1 CAUTI risk factor – the indwelling catheter.2
73% CAUTI rate reduction3
For adolescent and adult patients with female anatomy4

Performs up to 24 hours4

20% estimated average savings with PrimaFit5

17 to 1 CAUTI reduction in a 150-bed facility6
Is your current practice adding unnecessary costs?
A nationwide survey of 30 hospitals using a competitive female external urine collection device revealed additional “hidden” costs that impact workflow and finances.
3.4
female external devices were used on average per patient day5
100%
used additional supplies (tape, mesh underwear, pads, etc)5
$2.91
average additional supply cost to change each device*
*Disclaimer: Supply cost ($2.91) calculated using the National average price for additional supplies shown (tape and mesh underwear) or more
The benefits of standardizing care
Now’s the time to standardize external urine management at your hospital with Sage PrimaFit and Sage PrimoFit+. Our goal is to help your staff streamline workflow and enhance efficiency while providing patients with comfortable, safe, care. In addition to our commitment to quality, we provide comprehensive training, in-servicing and supplemental educational opportunities. This focus on support means staff can confidently utilize both devices to their full potential, which may help drive improved outcomes for more patients.
“Stays on very well and keeps them super dry and clean!”
– Clinician at an acute care hospital using Sage PrimoFit+7*
*Disclaimer: The product used was PrimoFit, but the comment is applicable to PrimoFit+.
“It fits very well on patients and it’s more comfortable.”
– Clinician at acute care hospital about Sage PrimaFit8
✓ Fits
✓ Stays
✓ Performs
Designed to perform – for more patients
Both devices are approved for adolescents and adults for up to 24 hours4 and both provide a customizable fit that stays in place and diverts urine away from the skin to keep your patients comfortable.
PrimoFit+ External Urine Management for the Male Anatomy (for adults and adolescents)
Customizable fit eliminates the need for sizing charts
Stays in place with adhesive pad and base adhesive
Can be left in place for up to 24 hours
PrimaFit External Urine Management for the Female Anatomy (for adults and adolescents)
Contours to patient anatomy and maintains shape
Gentle adhesive pad keeps the device in place
Can be left in place for 12-24 hours
Interested in learning more? Connect with an expert.
Learn more about reducing CAUTIs
Strategies to prevent Catheter-Associated Urinary Tract Infections (CAUTIs)
The Compendium offers updated guidance for hospitals to help reduce the risk of catheter-associated urinary tract infections (CAUTIs).
Learn moreSage PrimaFit External Urine Management System for the Female Anatomy helped reduce CAUTI rate 63%
Catheter-related infections decreased from before the introduction to EUDFA and after. This is not a cause-and-effect relationship, but the trend is promising.
Learn moreReduce the risk of catheter-associated urinary tract infection (CAUTI) in hospitals
Catheter-associated urinary tract infections (CAUTIs) are among the most common types of healthcare-associated infections, and reducing them is beneficial to hospitals, caregivers and patients.
Learn moreNYC Medical Center Implementation of External Female Catheter avoids $16M in costs over 2 years
In 2018, the implementation of the external female urine management device was introduced as a standard of nursing care as an alternative to an indwelling urinary catheter. Data captured in 2019 and 2020 showed utilization of the external female device to be 36% and 46%, respectively.
Learn moreReferences:
1. Loveday HP, Wilson JA, Pratt RJ, et al (2014) epic 3: national evidence-based guidelines for preventing healthcare associated infections in NHS hospitals in England. J Hosp Infect 86(Suppl 1): S1–S70.
2. Centers for Disease Control and Prevention, Catheter-associated Urinary Tract Infections (CAUTIs). Available at https://www.cdc.gov/hai/ca_uti/uti.html. Accessed August 24, 2017.
3. Beeson T, Davis C, Vollman K, Chasing Zero Catheter Associated Urinary Tract Infections (CAUTIs) through Implementing a Novel Female External Urine Collection Device in a Tertiary Academic Surgical Intensive Care Unit (SICU) Presented at APIC 2018.
4. Sage PrimaFit Instructions for use (IFU) 678907, 2023 and Sage PrimoFit+ Instructions for use (IFU) 843801, 2024.
5. Data on file, Sage Products LLC, Results pulled from 30 facilities total cost of ownership survey data collection. Retrieved August 8, 2022.
6. Data on file, Sage Products LLC. CMR 29283 CustomerOne Report
7. Data on file, Sage Products LLC, Product Evaluation C1 Summary 200911.
8. Data on file, Sage Products LLC, Product Evaluation C1 Summary 230328.
SAGE-UM-SYK-1231833_REV-0_en_us