Hip and knee replacement
Our joint replacement solutions offer you the combination of market-leading implants for the hip, knee, ankle and shoulder along with Mako Robotic-Arm Assisted Technology consultative services for the orthopaedic service line.
Upper extremity
Our trauma and extremities portfolio offers you market-leading implants for the treatment of long and small bone fractures, as well as extremity joint replacements.
Sports medicine
Motion regained. Life renewed. We are driven to lead the advancement of sports medicine, and we deliver a wide range of innovative sports medicine implants, instrumentation, resection and biologic solutions. Our solutions focus on minimally invasive and open approaches to the shoulder, hip, knee and small joints.
Foot and ankle
We are dedicated to helping foot and ankle surgeons treat their patients more efficiently while enhancing patient care and the overall healthcare experience. Constantly driven to innovate, we offer a diverse array of advanced medical technologies and a comprehensive portfolio of products.
ENT
Our dedication to over 20 years of research and development of surgical ENT products reflects the heritage that has made us a world leader in medical technology.
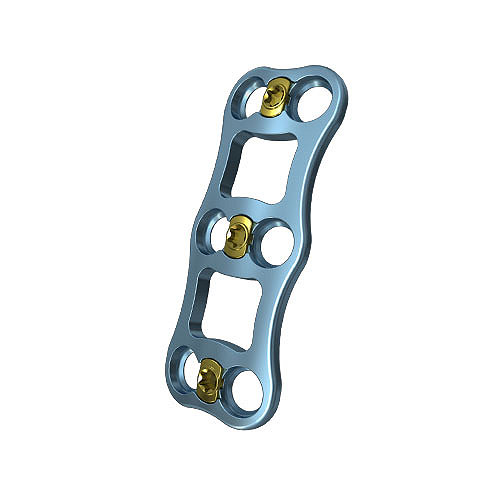
Spine
Our continually expanding portfolio offers spinal solutions spanning from the occiput to the pelvis.
This document is intended solely for the use of healthcare professionals. A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery.
The information presented is intended to demonstrate the breadth of Stryker’s products. A surgeon must always refer to the package insert, product label and/or instructions for use, including the instructions for cleaning and sterilization (if applicable), before using any Stryker product. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Stryker representative if you have questions about the availability of Stryker products in your area.
MKOSYM-WC-27_26105; GSNPS-WC-14_15227; GSNPS-WC-14_15227; Literature Number: 1000902691 Rev A; OT-AWI-42, 08-2017; 9100-004-801 Rev. None; GEN-WB-2_15463, D0000022844