EZout Powered Acetabular Revision System
25-Jan-2021
The current state of hip replacement revision surgery is often, in a word, medieval, in that traditionally manual tools such as curved osteotomes or manual cup removal systems are used.
There are over 45,000 acetabular revisions performed each year in the United States.1 In Europe, there are 183,600 large joint reconstructive implant surgeries.2 For many orthopaedic surgeons, these procedures can be time-consuming and physically exhausting.
For surgeons who typically perform many such surgeries, an updated acetabular revision system could make acetabular revisions easier to perform and be a great benefit.
In this article, we’ll discuss the current state of acetabular revision surgery and the way that the EZout system can help make the surgical process more efficient and less physically draining.

The acetabular cup removal procedure has been performed in a way that requires a hammer to be used to pound a long metal handle with a curved blade into the peripheral bone surrounding the cup.3 This process has to be repeated several times in order to loosen the bone around the implant and effectively remove it.
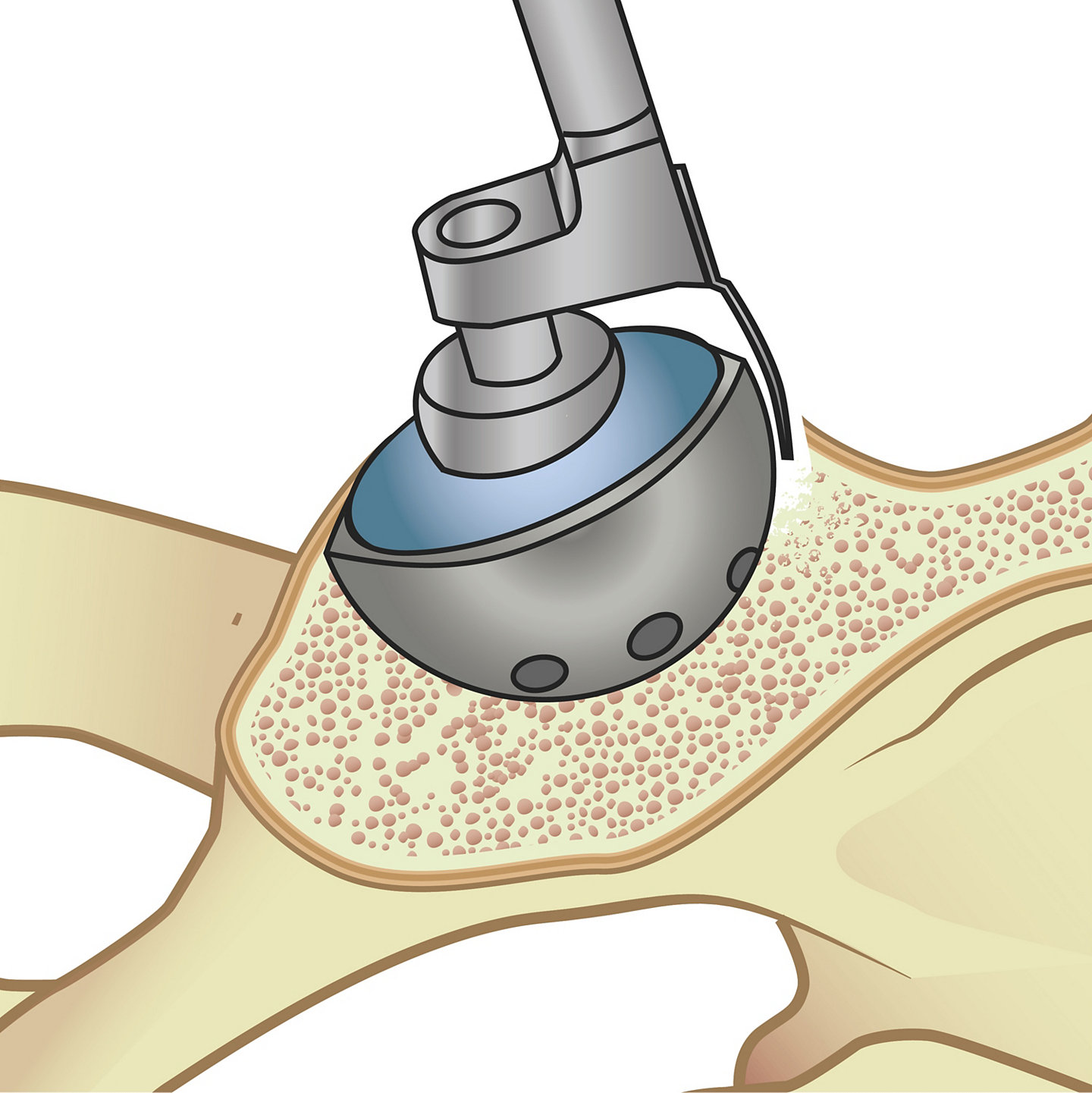
But even the strongest doctor will have to exert substantial physical energy while hammering in order to carve out the space between the pelvic bone and the implant.
Similarly, the long L-shaped handle of the removal tool, which needs to be rotated 360 degrees during the surgery to ensure full separation of the cup from the bone, requires a large workspace in order to complete the procedure.
One of the main risks in traditional hip revision surgery using manual tools is unintentionally removing the cup with large amounts of bone residue still in tow.5
Another, much more serious risk, is the potential of cracking the pelvis bone while trying to extract the acetabular cup. The amount of force that’s required in a traditional revision surgery can be high, and this, coupled with poor or weakened bone strength or arthritis, can lead to an intraoperative fracture.6
Also, not to be ignored, is the fact that surgeons are the ones required to expend the energy in removing the cup from the patient’s hip and performing the replacement. As has been mentioned, this procedure often requires a significant amount of energy and can leave surgeons feeling physically exhausted and burnt out.7
Advancements in surgical technology, especially in orthopaedics, could allow surgeons to improve their operations without expending as much physical effort as would otherwise be required.
Although there are always risks with hip revision surgeries, EZout can help alleviate some of these commons risks as demonstrated below.

*Stryker literature number 100-004-047 Rev A
Stryker’s EZout powered acetabular revision system is a power saw attachment for the Stryker’s System 8 power tool system, which is intended to cut bone and hard tissue around the bone-to-cup interface. The system incorporates a power tool (handpiece), attachment, plugs, riser rings, sizing equipment, and a variety of blades to aid in the removal of a hemispherical acetabular cup during revision of hip arthroplasty.8
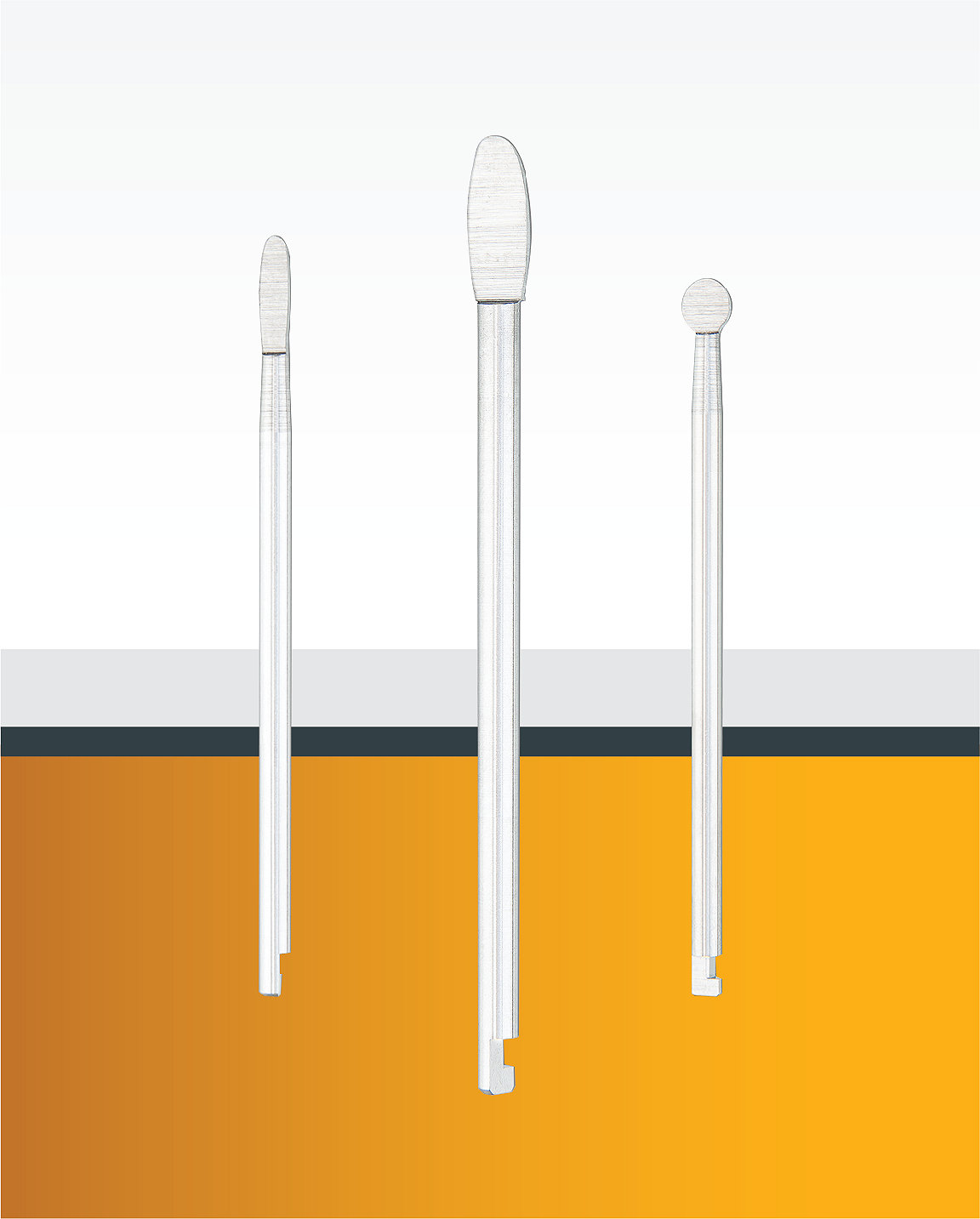
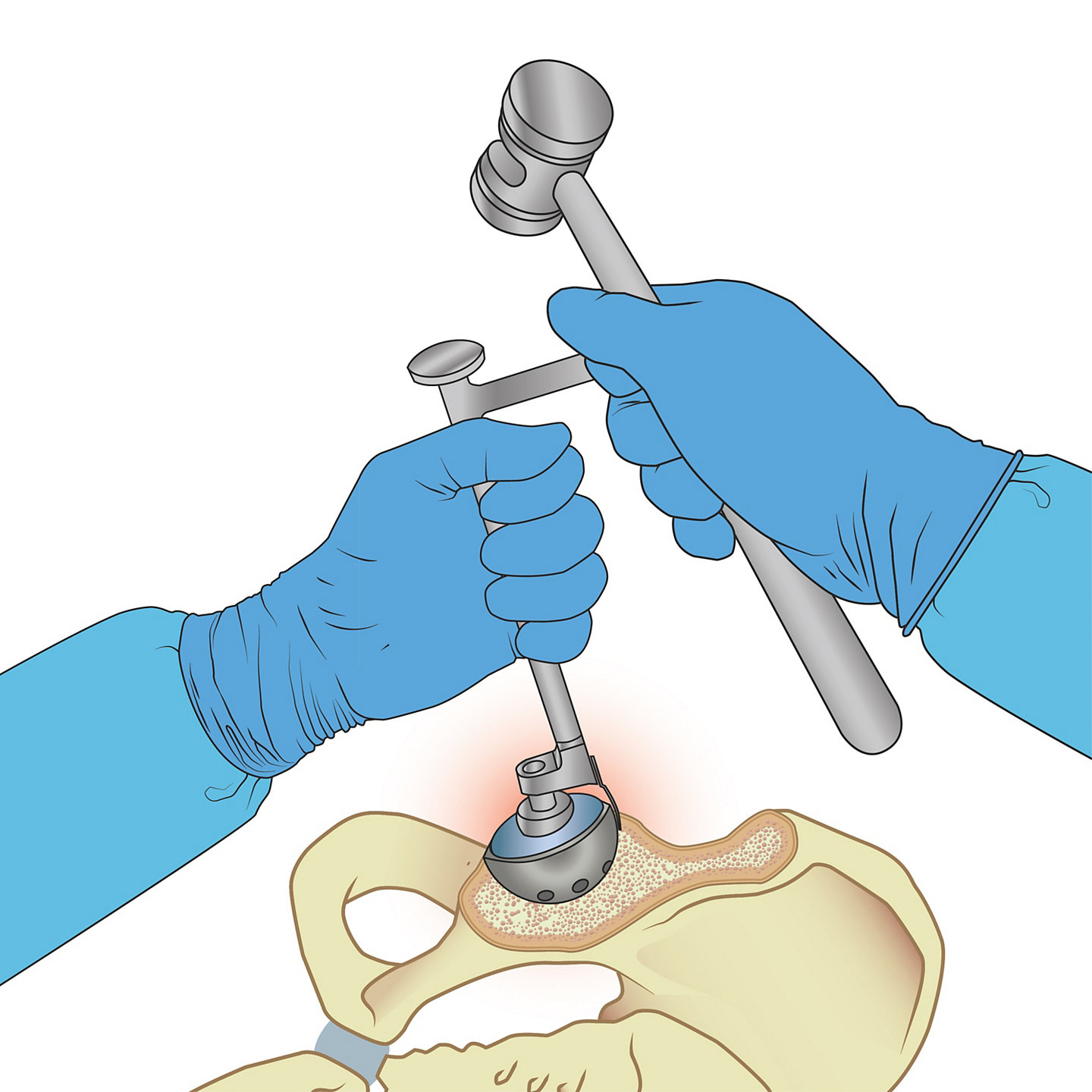
In order to carry out the acetabular cup removal as quickly and accurately as possible, the EZout system features a four-step process: (1) Remove the liners and screws of the cup, (2) Use the Stryker measuring tool to size the centering cup, (3) Size the blade according to the outer diameter of the cup (Figure A), and (4) begin removing the cup by pulling the attachment handle toward the handpiece to fully retract the blade, inserting the attachment into the centering plug, and engaging the trigger to oscillate the short blade with the bone by gently sliding the attachment handle toward the cup while vigorously rotating the handle back and forth (Figure B), and switching to the long blade (designed to reach around the entire underside of the cup) to free the cup, once the short blade has successfully made its way around the circumference of the cup.
Step 1
Remove the liner/screws
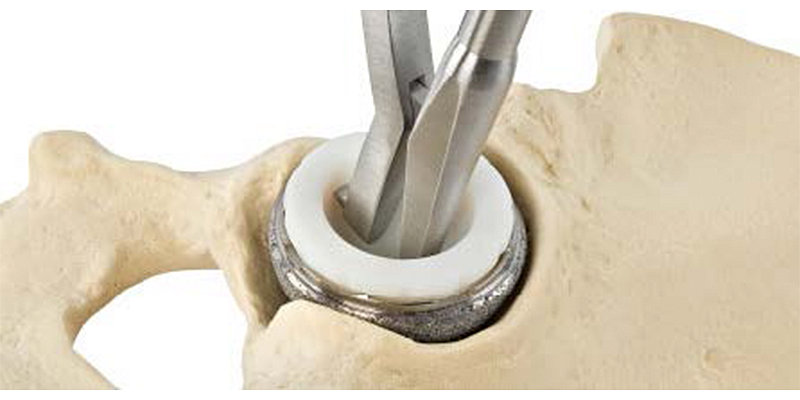
Also, identify whether any fins or spikes are present and will need to be cut around.
Step 2
Size the centering plug

The centering plug size is determined by the outer diameter of the liner. Use the plug sizing template to select and install the appropriate centering plug.
Step 3
Size the blade

The blade size is determined by the outer diameter of the cup. Determine the blade size by comparing the edge of the implanted cup to the blade sizing tool.
Figure A9
Note: The green indicator ring will be flush with the back of the attachment to confirm the full depth of the blade.
Step 4
Remove the cup

Pull the attachment handle toward the handpiece to fully retract the blade and insert the attachment into the centering plug.
Centre

Fully retract the blade, then engage the trigger to oscillate the blade. Engage the blade with the bone by gently sliding the attachment handle toward the cup while vigorously rotating the handle back and forth.
Step 3
Size the blade

Always verify that the short blade can move freely around the entire circumference of the cup before switching to the long blade. Ensure the green indicator ring is flush with the back of the attachment to confirm full depth.
Figure B9
In a study comparing the efficiency and efficacy of the Zimmer explant acetabular removal system with the Stryker’s EZout, a surgeon performed acetabular cup removal with both tools to see which was more efficient at removing the cup in terms of time and effort, as well as which required less force and temperature.10
Six foam models were used in total for in vitro testing; three models were designated for testing with the EZout system, and three were tested with the Zimmer explant system. The models were made of foam with a density of 0.48 g/cc, resembling that of a human pelvic bone.
The study found that the EZout system required an “overall lower force and torque” and produced “lower strains in the surrounding composite bone.” The same study also found that the EZout required less exerted force from the surgeon in the removal of the cup and less overall time complete.
The EZout system brings a new technological advantage to the operating room. Surgeons and hospitals can benefit from adopting this new technology.
[1] Gwam CU, Mistry JB, Mohamed NS, Thomas M., Bigart KC, Mont MA, Delanois RE. Current epidemiology of revision total hip arthroplasty in the United States: National Inpatient Sample 2009 to 2013, J Arthroplasty 32, no. 7 (July 2017) 2088-2092, https://www.sciencedirect.com/science/article/pii/S088354031730164X.
[2] https://pdf.medicalexpo.com/pdf/zimmer/explant-acetabular-cup-removal-system-surgical-technique/74894-120929.html.
[3] Kwong, L., Billi, F., Keller, S., Kavanaugh, A., Luu, A., & Paprosky, W. (2019). A Comparative Study Between the Stryker EZout Powered Acetabular Revision System and the Zimmer Explant Acetabular Cup Removal Systems. Techniques in Orthopedics, 1. doi: 10.1097/bto.0000000000000409
[4] Cedars Sinai. Revision hip surgery, accessed Dec. 18, 2019, https://www.cedars-sinai.org/health-library/diseases-and-conditions/r/revision-hip-surgery.html.
[5] Ibid.
[6] Ames SE, Cowan JB, Kenter K, Emery S, Halsey D. Burnout in orthopedic surgeons: A challenge for leaders, learners and colleagues: AOA critical issues, J Bone Joint Surg Am. 2017 Jul 19;99(14):e78, https://www.ncbi.nlm.nih.gov/pubmed/28719565.
[7] https://www.strykermeded.com/media/2649/ezout-technique-guide.pdf
[8] Kwong L., Billi F., Keller S., Kavanaugh A., Luu A., Paprosky W. A comparative study between the Stryker EZout powered acetabular revision system and the Zimmer explant acetabular cup removal systems, 1-8,
[9] Ibid, 6.
[10] Ibid, 1-8.
This article is intended solely for the use of healthcare professionals. A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that healthcare professionals be appropriately trained in the use of any particular product before use.
The information presented is intended to demonstrate the breadth of Stryker product offerings. A healthcare professional must always refer to the package insert, product label and/or Instructions for Use before using any Stryker product. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Stryker representative if you have questions about the availability of Stryker products inyour area. Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: Precision, Stryker. All other trademarks are trademarks of their respective owners or holders. The products depicted are CE marked in accordance with applicable EU Regulations and Directives.
Stryker © 2021
D0000023496 Rev AA / 2021-28367 / SMACC 2020-28304