Stryker Interventional Spine is driven to make healthcare better. We provide an extensive and a tailored product portfolio for vertebral augmentation and radiofrequency ablation procedures, along with a diagnostic tool and decompression treatment for contained disc herniations.
Our team delivers high quality personalized education programs designed to advance minimally invasive surgical techniques. You can count on us to support your success and drive value through training focusing on the correct clinical and technical application of our products and procedures.
From the office to the operating room and every stop in between, we are here to serve you with a portfolio built on innovation and a legacy of customer service.
Our services and product categories
Investigator Initiated Study
Stryker Interventional Spine provides support in the form of monetary funding or supply of product(s) to Investigator Initiated Study (IIS). IIS are independent clinical research projects that are conceived, initiated, conducted, and sponsored by a qualified third-party investigator (Sponsor Investigator).
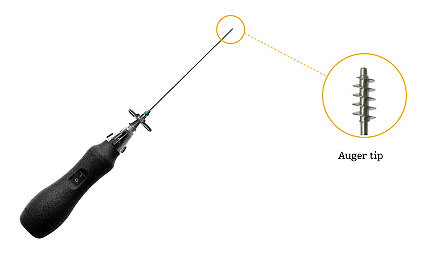
Learn morePercutaneous Disc Decompression System
The Stryker Dekompressor disc removal system is intended for use in aspiration of disc material during percutaneous discectomies in the lumbar, thoracic, and cervical regions of the spine.
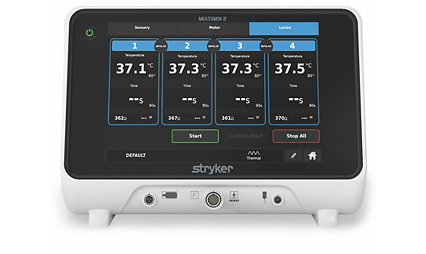
Learn moreMultiGen® 2 Radiofrequency Generator
Enter the next generation of radiofrequency nerve ablation and discover the control and confidence that the MultiGen® 2 Radiofrequency Generator offers. Together, the MultiGen® 2 RF Generator and the Venom cannula and electrode system deliver large lesions in a short amount of time. Our Performance Platform offers a lot for you and your patients.
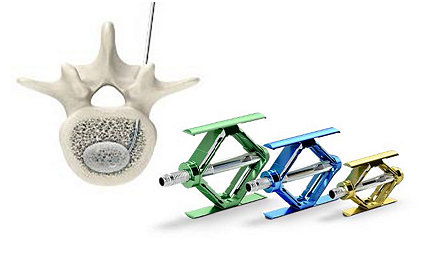
Learn moreVertebral compression fractures
Stryker Interventional Spine offers a broad portfolio of products for VCF treatment. Our product portfolio, which includes the SpineJack® System, the iVAS® balloon system, the AVAflex® vertebral balloon system, mixer and delivery systems, bone cements, bone biopsy and needles, lets you customize your treatment approach.
Learn moreSMACC 2023-34397