LIFEPAK 15
High-performance defibrillation
Trust proven clinical performance
The LIFEPAK 15 monitor/defibrillator delivers up to 360J, which has been shown to improve conversion rates for difficult-to-defibrillate patients.1-4 Elevate STEMI care with the industry-leading University of Glasgow ECG Analysis Program and ST-segment trend monitoring, which continuously monitors all 12-leads, alerting you to changes.
Stay focused in chaotic settings
From intuitive controls to a dual-battery system and a large 8.4-inch anti-reflective color display that changes to high-contrast mode with a single button, the LIFEPAK 15 device helps you work more efficiently, no matter how chaotic the situation becomes.
Depend on rugged durability
With more than a dozen durability enhancements from the previous generation of LIFEPAK devices, the LIFEPAK 15 device is our toughest yet – built to withstand drops, shocks and extreme vibration.
Continually improve team response
The LIFENET System connects the LIFEPAK 15 device through the cloud to transmit emergent patient data to the hospital. This enables ease of documentation, care team activation for time-sensitive emergencies and valuable insights that support continuous performance improvement throughout the patient journey.
Simplify device management and maintenance
Help ensure the device is always ready to go with two reliable batteries and a daily self-test that alerts you to possible issues before you experience them in the field. Easily track device health and readiness via the LIFENET Asset management system.
PRODUCT HIGHLIGHTS
Your trusted partner wherever the next call takes you
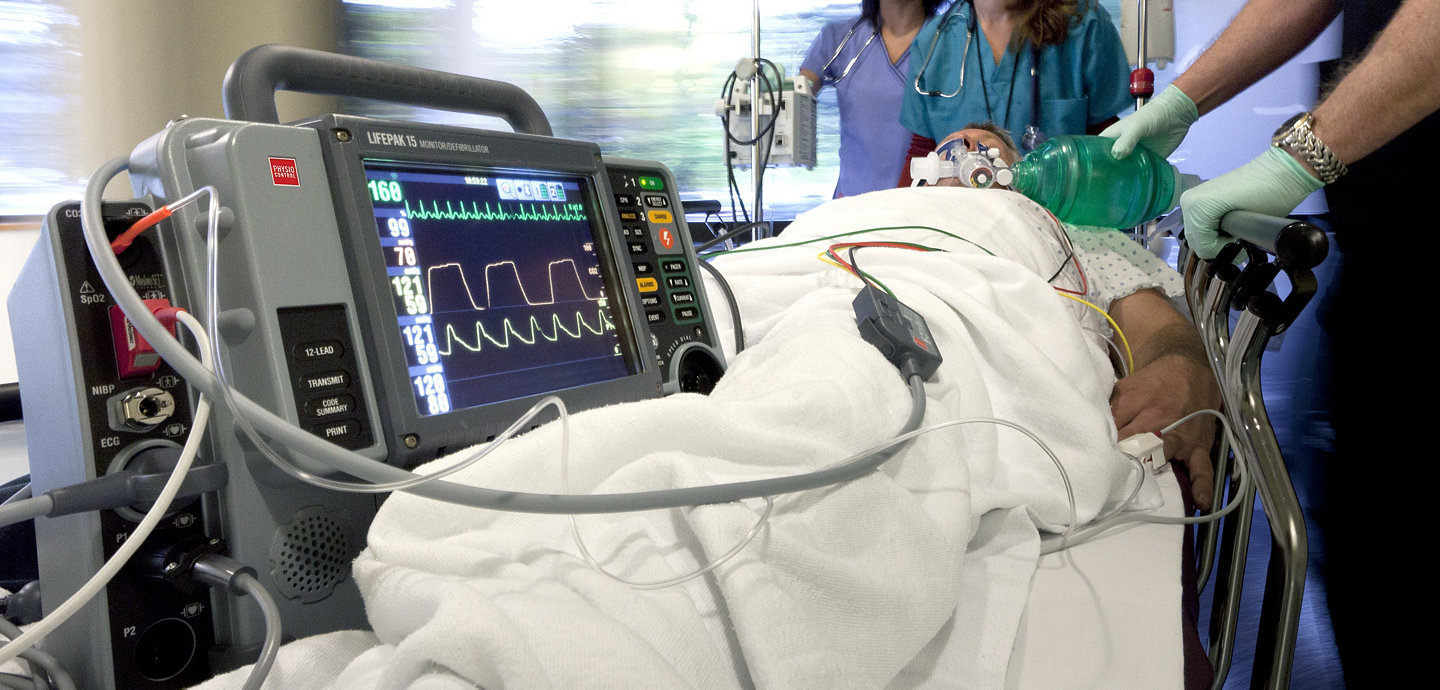
Easy to use so care teams can focus on patients
- Dual-mode display screen provides maximum visibility—59 percent larger screen than competitive devices1
- All key functionality with a single button push
- Easy access to cables, connections and printer
Supports improved resuscitation results
- Proven CPR guidance—shown to guide responders to perform compressions at 100/minute and avoid over-ventilation2
- Capture all displayed waveforms and CPR quality for post-event review
- The highest escalating energy available, up to 360J biphasic, for difficult-to-defibrillate patients
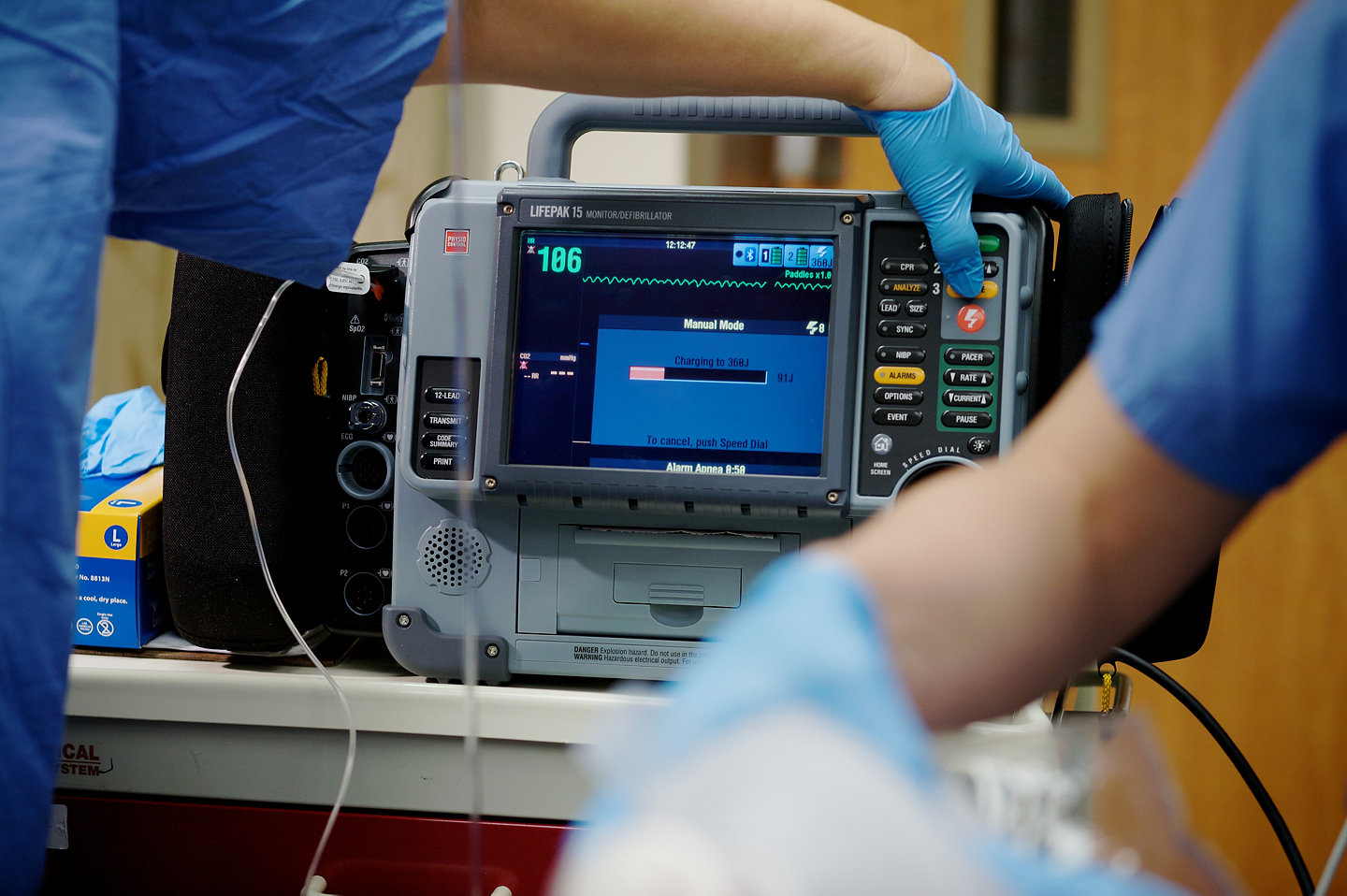

Improves operational effectiveness
- Upgradeable platform adapts to evolving protocols and new care guidelines
- Instant access to device health and readiness data through LIFENET Asset
- Enhances team review with rapid data export to CODE-STAT post-event review software for QA/QI
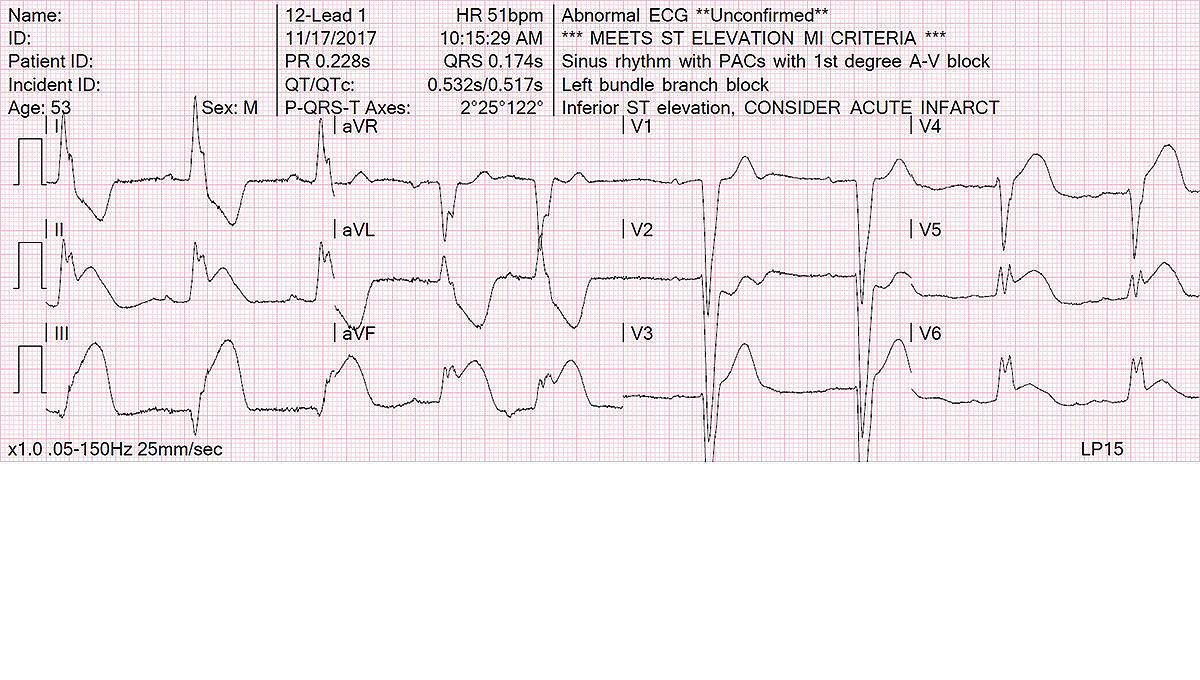
Uses a top 12-lead ECG algorithm
- Trusted, proven University of Glasgow 12-Lead ECG Analysis Program
- Continuous monitoring of all 12-leads with alerts when significant ST segment changes are detected
- Uses Sgarbossa criteria and measures STJ at the J point for possible STEMI patients and provides pediatric interpretation for pediatric patients

LIFEPAK TOUGH
- Corner guards, shock-absorbing handle and reinforced cable connections
- Dual-layer, anti-scratch screen
- Withstands severe impacts and extreme vibration
FAQs
The basic configuration of the LIFEPAK 15 with a new roll of paper and two batteries installed weighs 17.5 pounds. The fully featured configuration with a new roll of paper and two batteries installed weighs 18.5 pounds.
Height: 12.5 inches
Width: 15.8 inches
Depth: 9.1 inches
AED mode - for automated ECG analysis and a prompted treatment protocol for patients in cardiac arrest
Manual mode - for performing manual defibrillation, synchronized cardioversion, noninvasive pacing and ECG and vital sign monitoring
Archive mode - for accessing stored patient information
Setup mode - for changing default settings of the operation functions
Service mode - for authorized personnel to perform diagnostic tests and calibrations
Demo mode - for simulated waveforms and trend graphs for demonstration purposes
Size (active viewing area) - 8.4 inches diagonal; 6.7 inches wide x 5.0 inches high
Resolution - 640 dot x 480 dot color backlit LCD
User selectable display mode - Users can choose between full color or SunVue display high contrast
Waveform display sweep speed - 25 mm/second for ECG, SpO2, and IP; 12.5 mm/sec for CO2
Up to three waveforms may be displayed at the same time.
Quick set - activates alarm for all active vital signs
VF/VT alarm - activates continuous (CPSS) monitoring in manual mode
No breath alarm - occurs when 30 seconds has elapsed since the last detected respiration
Heart rate alarm limit range - upper: 100 - 250 bpm; lower: 30-150 bpm
The LIFEPAK 15 uses a lithium-ion battery. Each battery has a fuel gauge that indicates its approximate charge.
Connect with an expert
Have feedback about Stryker’s products and services? Visit our product experience page to connect with us.
M0000010305 REV AA
SERVICE AND SUPPORT
Robust support for your team
Technical support
We'll work with you to quickly assess your situation and find the best solution.
ProCare service plans
Focus on saving lives while extending the integrity of your devices with a ProCare service plan.
Product resources
Find a variety of documents including best practices, datasheets and clinical information for easy download.
Training and education
Discover webinars, online and in-person courses and other resources to strengthen your clinical education.
Related products
LUCAS 3, v3.1 chest compression system
Deliver high-performance, continuous chest compressions with less strain and caregiver risk.
Learn more1. Physio-Control internal observation (Jan 2015)
2. Kern KB, Stickney RE, Gallison L, Smith R. Metronome improves compression and ventilation rates during CPR on a manikin in a randomized trial. Resuscitation. 2010;81:206-210
M0000010067 REV AA










