Search Results
Showing results for: additive manufacturing
Showing 50 Of 263 records
-
Tritanium C
Anterior Cervical Cage
A hollow implant that consists of a unique configuration of both solid and porous structures built using AMagine Technology, our proprietary approach to implant creation using additive manufacturing
-
Tritanium TL
Curved Posterior Lumbar Cage
A hollow implant that consists of a unique configuration of both solid and porous structures built using AMagine Technology, our proprietary approach to implant creation using additive manufacturing
-
Tritanium PL
Posterior Lumbar Cage
A hollow, rectangular implant that consists of a unique configuration of both solid and porous structures built using AMagine Technology, our proprietary approach to implant creation using additive manufacturing
-
Connected OR operating system
OR integration platform
Our OR integration, with its intuitive workflow and safety features, allows nurses to focus on delivering the highest level of patient care rather than managing the nuances of the surgical space.
-
Viju Menon
Group President, Global Quality and Operations
Viju Menon was named group president, global quality and operations in April 2018.
-
Acetabular System
Acetabular System
Have confidence in a powerful combination–a system that builds on the legacy that has defined our Trident brand for more than two decades, paired with the latest additive manufactured Tritanium In-Growth Technology or PureFix HA.
Trident II is created for case-to-case confidence that comes from building on a system backed by years of successful clinical data1-5 and reliable features such as the notable Trident Innerchange Locking Mechanism, Modular Dual Mobility (MDM), and our X3 precisely engineered polyethylene. -
Triathlon® Cementless – Fixation you can trust.
Fixation you can trust.
Triathlon Cementless combines the kinematics of Triathlon with the latest highly porous biologic fixation technology. With over one million cementless knees implanted to date1 and impressive survivorship data2-4 since it was introduced in 2013, Triathlon Cementless has become a trusted solution for surgeons across the globe.
-
Global quality
At Stryker, quality is first in everything we do. Read our Quality Policy and learn more about how we build quality into our culture, our processes and our products.
-
Knee
Implants
We offer knee replacement implants for partial and total knee arthroplasty for primary and revision procedures. Our implants feature our flagship cemented and cementless TKA solution, the Triathlon Knee System.
-
Spine
We deliver the total spine experience, empowering our customers to keep the world moving. Together, we do more to challenge the standard, respond to clinical needs and pioneer new possibilities.
Our core technologies include biologic solutions, spinal implants, and surgical instruments for use in spine procedures. Our enabling technologies include digital health, guidance, and imaging for both cranial and spine procedures.
-
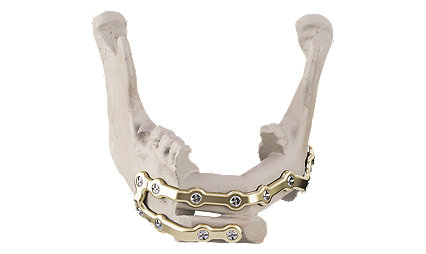
Facial iD - Mandible Reconstruction
A patient specific solution
Rigid internal fixation for primary and secondary mandible reconstruction.
-
Triathlon® Hinge
Introducing the newest addition to the Triathlon Knee Revision System
Triathlon® Hinge, the newest addition to the Triathlon Revision Knee System, has been designed to be a single platform for surgeries in patients with significant bone loss and/or ligament deficiencies.
-
CR Reports and Resources
ESG Resource hub
Access Stryker’s environmental, social and governance reports, data and resources
-
Monterey AL Interbody System
Fixated on science
With a design to enhance the ability to create indirect decompression, a Tritanium structure to facilitate bone in-growth [1], and an array of footprints, heights and lordotic options — our Monterey AL Interbody System aims to deliver on all fronts. Plus, our instrumentation is designed to support your surgical style whether it’s freehand, partially guided or fully guided. Here’s a look at the careful crafting we do to help meet your surgical needs and personal preferences.
-
Environmental programs
Climate and the environment
Environmental programs, processes and initiatives to drive sustainability
-
We move lives
Together, we move lives and help get patients back to the activities they love.
-
Prime Series | Electric hospital stretchers
Stryker's hospital stretchers
Our Prime Series stretchers are designed to reduce physical strain on clinicians, enhance comfort during patient transport and drive hospital efficiency. Fully equipped and highly configurable, our Prime Series stretchers allow you to create a solution that meets your specific needs.
-
Direct Superior Approach
Superior soft tissue preservation
Direct Superior approach is an iliotibial band and muscle-sparing surgical approach to the hip that offers the potential benefits to the patient of faster recovery, less pain and greater satisfaction.
-
Champion SlingShot
Single Portal Suture Passing System
The Champion Slingshot has been optimized for use in the shoulder and allows you to both pass and retrieve suture using a single portal. Its ergonomic thumb-slide design helps to eliminate additional procedural steps and potentially reduce operating time.
-
Pivot Guardian total hip system
State-of-the-art surgical table accessories spanning six hip procedures
The Pivot Guardian total hip system includes the Pivot Guardian post-free distraction system for hip arthroscopy and now expands your options to five additional hip procedures with additional state-of-the-art table accessories.
-
Operon D830
Surgical table
The Operon D830 is designed to deliver on the increasing demand for flexibility in your operating room.
-
Emergency Care training and education
Discover on-demand webinars, online and in-person training and additional resources created specifically for EMS, first responders, hospital clinicians, the community and more.
-
Start using PrimaFit – a urine management solution designed to stop leaks and limit CAUTIs
If your current device leaks – it’s not a urine management solution
PrimaFit is designed to help improve operational efficiency and reduce costs. Traditional indwelling catheters can cause discomfort, skin injury and infection.


















?$preset_426_254$)