Message from Professor Fares H. Haddad
University College London Hospitals - Princess Grace Hospital, London, UK

As a large academic center, we take every aspect of our patient pathway for total hip and total knee patients seriously. The introduction of Mako Total Knee has made a significant difference to our practice thus far. Preliminary data shows considerable advantages to our patients in terms of pain after surgery and in the speed of their recovery.
In this day and age total knee arthroplasty is a tremendous operation, allowing patients to return to a high level of activity.1,2 But not everybody has an easy recovery from their knee arthroplasty and a proportion of patients are still dissatisfied. Our experience of introducing robotic-assisted total knee arthroplasty has been favorable in that the three-dimensional CT-based planning allows us to understand the patient’s anatomy better. 3D CT-based planning, in conjunction with the haptic boundaries, also allow us to address the patients’ needs by fine-tuning the implant position and bony cuts in order to restore soft tissue balance. We are therefore able to undertake a patient specific procedure using tested implants like the Triathlon.
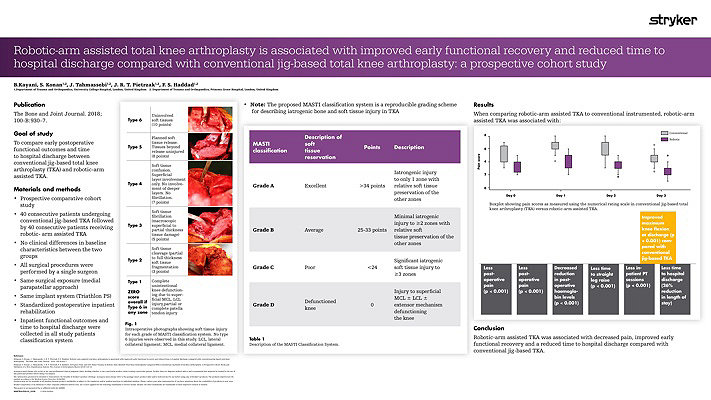
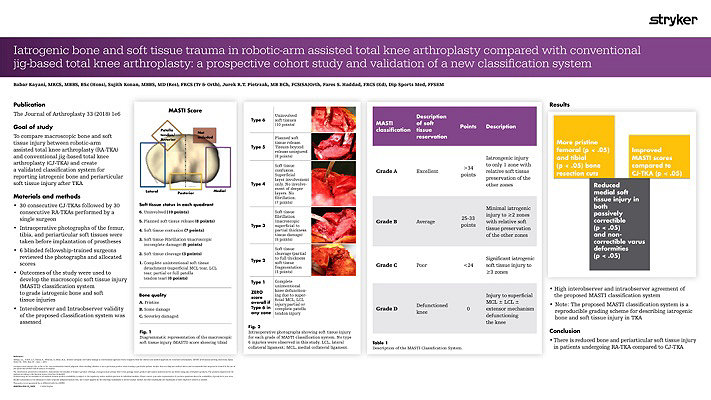
Our workflow continues to evolve and adapt. We are currently undertaking randomized prospective studies to evaluate the effects of using a Mako robotic-arm compared to using standard manual techniques in experienced hands. We are also looking at the effects of anatomical versus neutral mechanical alignment. Our pilot data shows less soft tissue injury after the use of Mako Robotic-Arm Assistance.3 We have also seen more precise bony cuts to plan.4 The reduction in soft tissue damage is perhaps partly related to the fact that the saw blade is not allowed to transgress beyond the haptic boundaries defined by the pre-operative plan.3 It is also important that we now do not have to do quite so many soft tissue releases as we can adjust the implant position in the plan to minimize those.5 Our preliminary data on this has been published. The MASTI score is now established and can be used by other investigators.3
We have seen that three-dimensional templating more accurately predicts implant sizes than two-dimensional templating,4 and also that the access to this technology has led us to use slightly smaller sizes of femoral components and to more reproducibly hit our target for a posterior condylar offset and tibial slope. We have seen a short learning curve. More importantly we have not seen a learning curve in terms of quality of outcome as the alignment, slope, joint line position and component positions achieved in the first ten cases were excellent compared to the pre-op plan.
The most striking results were that early recovery has been quicker compared to a comparative cohort of manual cases. We have seen lower pain scores, less usage of opiate analgesia, a shorter time to straight leg raising and a need for fewer physiotherapy sessions. The patients were able to go home approximately a day earlier and had a greater range of motion when they do so.5
Thus far our experience with Mako Total Knee has given us increased confidence in planning and in execution. This technology provides immediate intra-operative feedback and flexibility to change implant positions.5 Our data showed that there was less soft tissue damage than with conventional jig-based techniques even in experienced hands.3 Our pilot data showed that there was less pain in the peri-operative period and a quicker early recovery.5 Moreover, we have found the use of the Mako Total Knee a tremendous teaching, training and research tool.
- Bourne, R; Chesworth, B; Davis, A; Mahomed, N; Charron, K.; Patient satisfaction after total knee arthroplasty who is satisfied and who is not? Clinical Orthopaedics and Related Research 21 October 2009.
- Kahlenberg, CH, Nwachukwu BU, McLawhorn AS, Cross, Cornell, CN, Padgett, DE. Patient Satisfaction after TKA; A Systematic Review. 2018) 14:192–201. Published online: 5 June 2018/ DOI 10.1007/s11420-018-9614-8 HSSJ.
- Kayani B, Konan S, Pietrzak JRT, Haddad FS. Iatrogenic Bone and Soft Tissue Trauma in Robotic-Arm Assisted Total Knee Arthroplasty Compared with Conventional Jig-Based Total Knee Arthroplasty: A Prospective Cohort Study and Validation of a New Classification System. The Journal of Arthroplasty 33 (2018) 2496-2501.
- Pietrzak JRT, Rowan FE, Kayani B, Donaldson MJ, Huq SS, Haddad FS. Preoperative CT-Based Three-Dimensional Templating in Robot-Assisted Total Knee Arthroplasty More Accurately Predicts Implant Sizes than Two-Dimensional Templating. J Knee Surgery. 2018 Aug 1. doi: 10.1055/s-0038-1666829.
- Kayani B, Konan S, J Tahmassebi, Pietrzak JRT, Haddad FS. Robotic-arm assisted total knee arthroplasty is associated with improved early functional recovery and reduced time to hospital discharge compared with conventional jig-based total knee arthroplasty; A prospective cohort study. Bone Joint J 2018;100-B:930–7.
- Kayani B, Konan S, Huq SS, J Tahmassebi, Haddad FS. Robotic-arm assisted total knee arthroplasty has a learning curve of seven cases for integration into the surgical workflow but no learning curve effect for accuracy of implant positioning. Knee Surgery, Sports Traumatology, Arthroscopy. 2018 Sep 17. doi: 10.1007/s00167-018-5138-5.
Fares Haddad, FRCS (Ortho) is a paid-consultant of Stryker.
The opinions expressed by Fares Haddad, FRCS (Orth) during this presentation are those of Fares Haddad, FRCS (Orth) and not necessarily those of Stryker.
Individual experiences may vary.
The data included in this newsletter was collected and is owned by the surgeon author of this data. The data was not collected by Stryker.
A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery.
The information presented is intended to demonstrate the breadth of Stryker's product offerings. A surgeon must always refer to the package insert, product label and/or instructions for use before using any of Stryker's products. The products depicted are CE marked according to the Medical Device Directive 93/42/EEC. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your sales representative if you have questions about the availability of products in your area.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: Mako, Stryker, Triathlon. All other trademarks are trademarks of their respective owners or holders.
MAKTKA-BUL-2_19637