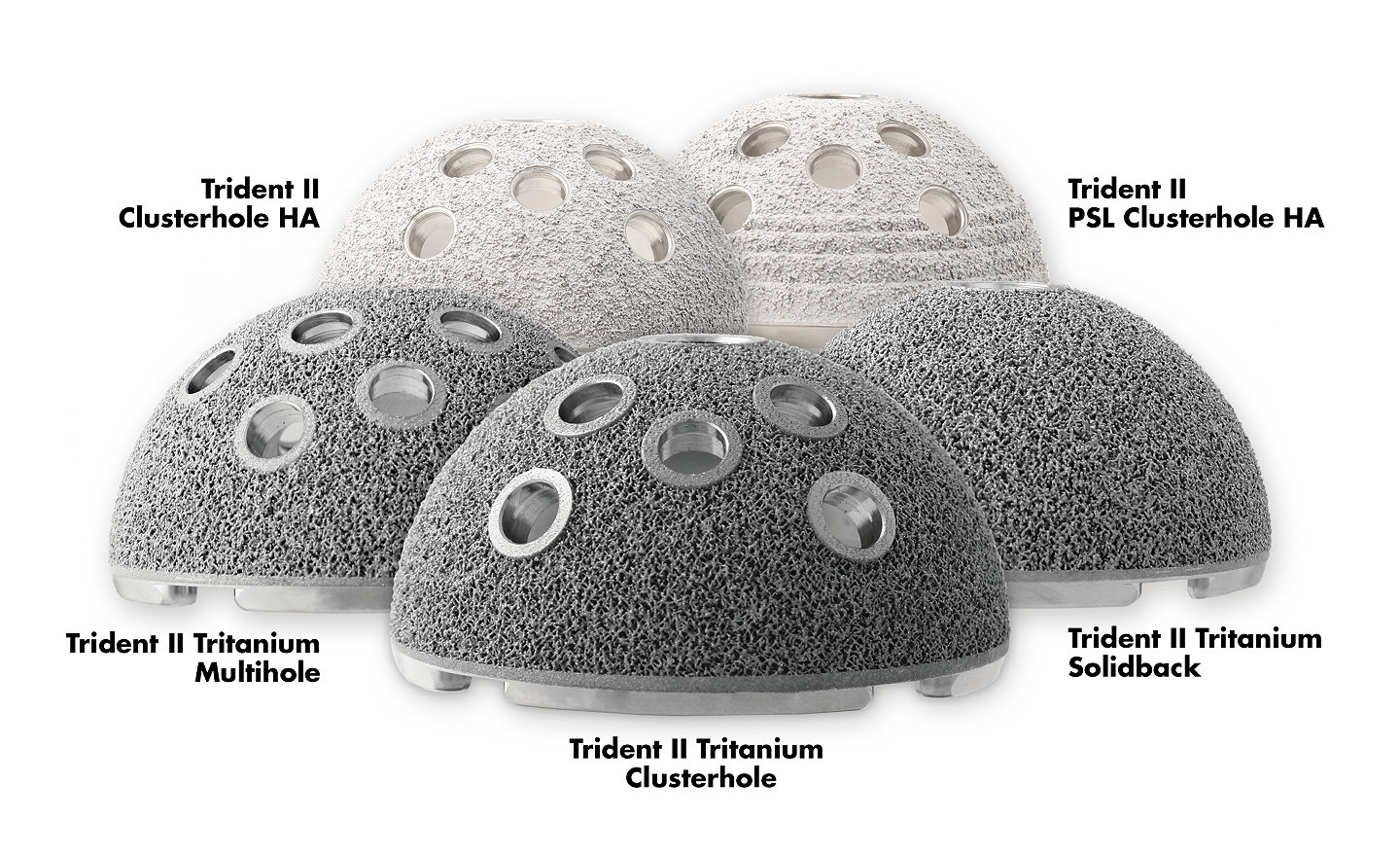
Trident II Acetabular System
Have confidence in a powerful combination–a system that builds on the legacy that has defined our Trident brand for more than two decades, paired with the latest additive manufactured Tritanium In-Growth Technology or PureFix HA.
Trident II is created for case-to-case confidence that comes from building on a system backed by years of successful clinical data and reliable features such as the notable Trident Innerchange Locking Mechanism, Modular Dual Mobility (MDM), and our X3 precisely engineered polyethylene.1-5
Key benefits
Get bigger, faster!6
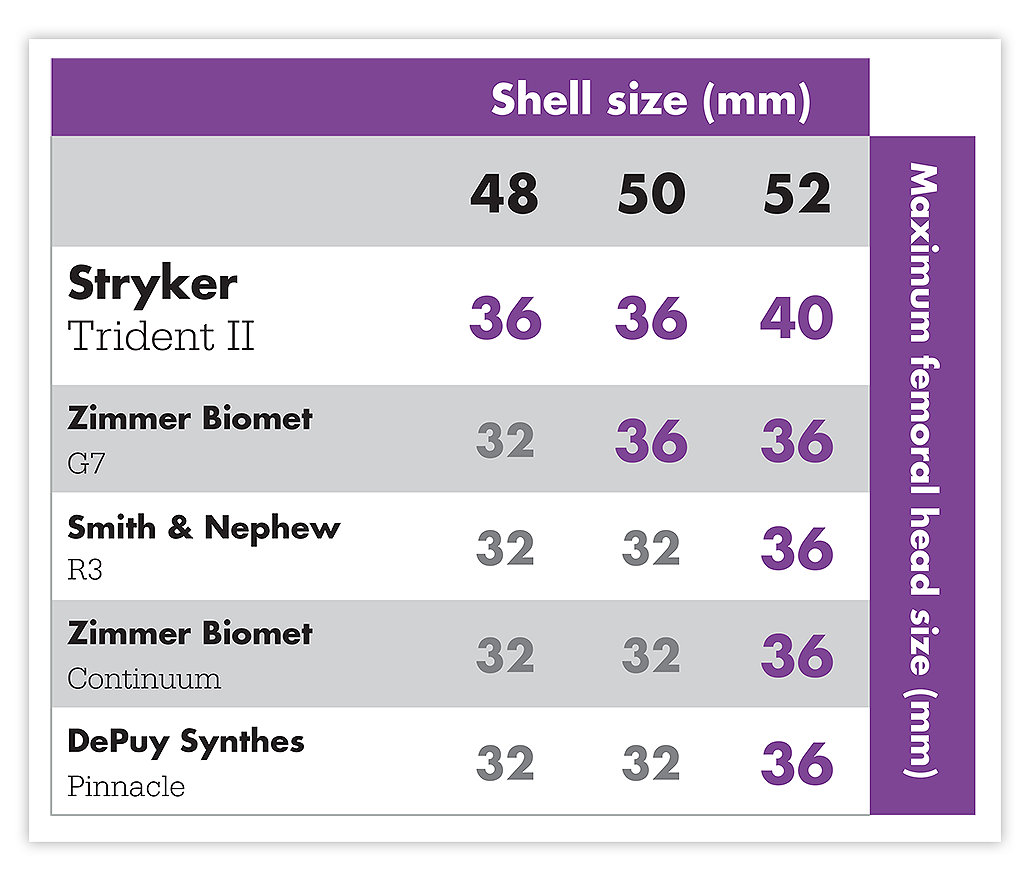
Slim Shell Wall
Trident II features a slim shell wall. This allows for large femoral head size options and optimal poly thickness to potentially aid in greater range of motion, joint stability and lower risk of dislocation.6,7,8

Additive Manufacturing
Trident II Tritanium features a controlled network of pores, designed to mimic the complex characteristics of cancellous bone, and to promote long-term biologic fixation.12
When it comes to fixation, have confidence in additive manufactured Tritanium.

PureFix HA
Stryker’s proprietary hydroxyapatite (HA) coating has been a consistent feature of our hip portfolio since it was first implanted in 1987. Trident II HA continues this legacy and provides surgeons the ability to select the fixation that best suits their patient by offering two options: a hemispherical or peripheral self-locking (PSL) shell.

Trident II leverages over 520+ CT scans* from Stryker’s SOMA (Stryker Orthopaedics Modeling and Analytics) technology. At the heart of SOMA is a large database of high resolution CT scanned bones from which size, shape, density, and inner and outer cortical boundaries can be drawn. Screw hole patterns for Trident II shells have been SOMA-verified to help achieve multiple screws in the safe zone of the acetabulum.13
*Based on 520+ CT scans
Instrumentation
From the hospital to the ASC, Trident II has you covered.
The streamlined rigid-container* compatible Trident II instrument trays can help reduce cost and complexity. Whether you choose to use the Mako system or manual instrumentation, Trident II instrument trays were designed with efficiency in mind to help free up space on your back table.
Two instrument trays, Core Reamers and General, are configured to address 99% of the patient sizes for implant bone preparation.14
*Trays compatible with Aesculap Configuration JN442 (base), JK48x (lid).


Hands free packaging15: Eliminate unnecessary contact to the porous surface of the implant prior to impaction.

Ball Joint Drill Shaft: Designed for soft tissue clearance and variable drill angulation while eliminating the open coil design found in other common drill shafts.

Keyed Shell Impactor: Designed to allow for simple shell attachment and quick adjustments to shell placement.
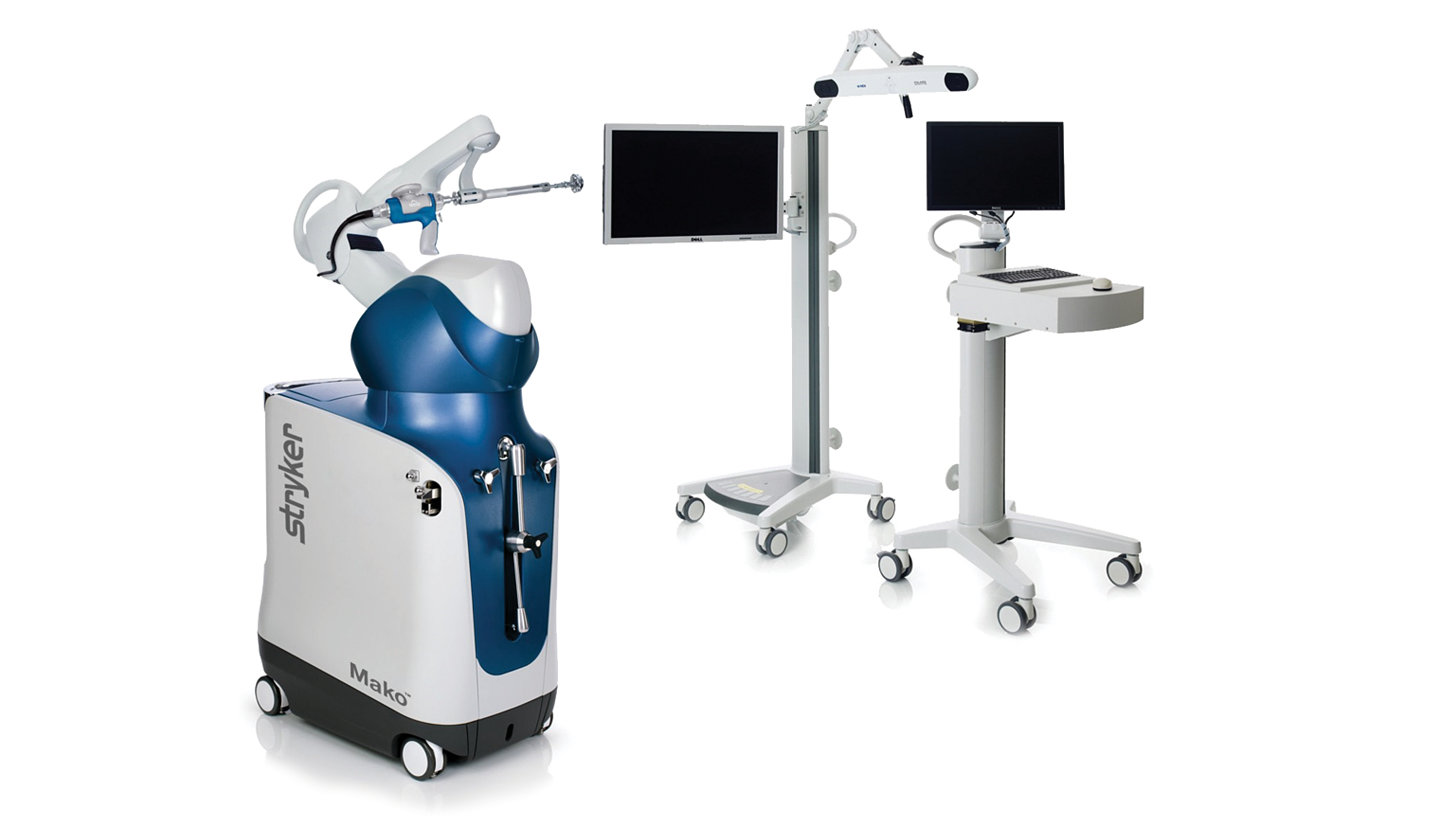
Combine the reliability of additive manufactured implants with the precision of Mako robotic-arm assisted surgery. Mako uses CT-based 3D modeling of the patient’s bony anatomy to provide you with a personalized surgical plan. Mako Total Hip enables surgeons to more accurately plan and place components, potentially reducing variability within the THA procedure and allowing for enhanced functional and clinical outcomes.16-23
Trident II Tritanium Acetabular Shells are created from the micron-level up using proprietary computer modeling software. Our unique additive manufacturing process builds each component layer by layer using a laser to fuse titanium alloy powder. This process enables the creation of a single shell with complementing porous and solid structures which eliminates the need for surface coatings. Every shell is built as a complete unit – this, along with numerous quality checks, helps to ensure that all implants are created with the highest level of quality assurance.
- Australian Orthopedic Association National Joint Replacement Registry, 2022 Annual Report.
- UK National Joint Registry, 2022 Report.
- D’Antonio J, et al. Second-Generation Annealed Highlight Crosslinked Polyethylene has Low Wear at Mean Seven Year Follow-up. Surgical Technology International. 2014 Nov;25:219-26.
- Jauregui J, et al. Dual Mobility Cups: an Effective Prosthesis in Revision Total Hip Arthroplasties for Preventing Dislocations. Hip Int. 2016 Jan-Feb;26(1):57-61.
- Su E, et al. The Role of Constrained Liners in Total Hip Arthroplasty. Clin Orthop. 2004;420:122–129
- Internal memo: Market Analysis of Poly Bearing Options - Trident II Versus Competitors. October 18, 2017.
- Burroughs B, et al. Range of Motion and Stability in Total Hip Arthroplasty with 28-, 32-, 38- and 44-mm Femoral Head Sizes In Vitro Study. The Journal of Arthroplasty, Vol. 20, No. 1, 2005 pp. 11-19
- Berry D.J., et al. Effect of Femoral Head Diameter and Operative Approach on Risk of Dislocation After Primary Total Hip Arthroplasty. J of Bone and Joint Surgery Vol. 87-A, No. 11 (2005); pp. 2456-2463
- Stryker R&D Technical Report: Characterizing the Physical Properties of the Trident II Tritanium Acetabular Shell. May 22, 2017. A0021722
- Stryker R&D Technical Report: Evaluation of the Coefficient of Friction of the Trident II Tritanium Surface. Sep 01, 2016. A0015751
- Stryker R&D Technical Memo: Trident II Tritanium Acetabular Shell Coefficient of Friction Equivalence Rationale. Oct 24, 2017. A0026809
- Stryker R&D Technical Memo: Comparison of Tritanium Porous Surface to Cancellous Bone. A0027625
- SOMA Screw Engagement. Stryker R&D Test Report - A0026638. October, 2017. SOMA verification at 45° inclination/20° anteversion.
- Sales data
- Internal memo: Nurse Appreciation Day VOC. July 28, 2017. A0029201.
- Nawabi D, et al. Haptically Guided Robotic Technology in Total Hip Arthroplasty: A Cadaveric Investigation. J Engineering in Medicine. 2012 Oct; 0(0) 1–8.
- Bragdon C, et al. A Multicenter Evaluation of Acetabular Cup Positioning in Robotic- Assisted Total Hip Arthroplasty. 43rd Annual Course: Advances in Arthroplasty, October 22-25, 2013, Boston, MA.
- Domb BG, et al. Comparison of Robotic-assisted and Conventional Acetabular Cup Placement in THA: A Matched-Pair Controlled Study., Clin Orthop Relat Res. 2014 Jan;472(1):329-36.
- Redmond J, et al. Does Robotic-Assisted Computer Navigation Affect Acetabular Cup Positioning in Total Hip Arthroplasty in the Obese Patient? A Comparison Study. J Arthroplasty. 2015 Dec;30(12):2204-7
- Redmond J, et al. The Learning Curve Associated with Robotic-Assisted Total Hip Arthroplasty. J Arthroplasty. 2015 Jan;30(1):50-4.
- Elson L, et al. Precision of Acetabular Cup Placement in Robotic Integrated Total Hip Arthroplasty. Hip Int. 2015 Sept;25 (6): 531-536
- Jerabek SA, et al. Accuracy of Cup Positioning, COR Restoration and Achieving Desired Hip Length and Offset with Robotic Total Hip Arthroplasty. 14th Annual CAOS Meeting, June 18-21, 2014, Milan, Italy.
- Thompson M. Efficient and Accurate Hip Length and Combined Offset with the MAKOplasty THA Femoral Express Workflow. Mako Surgical Corp. March 2014
TRIDII-WC-3_34344
