
Rest easy with Restoration® Modular
Make the most of your revision cases with a clinically-backed platform that is designed to leverage versatility and simplicity to help you achieve desired revision THA outcomes. Keep up with your active patients and rest easy with Restoration Modular.
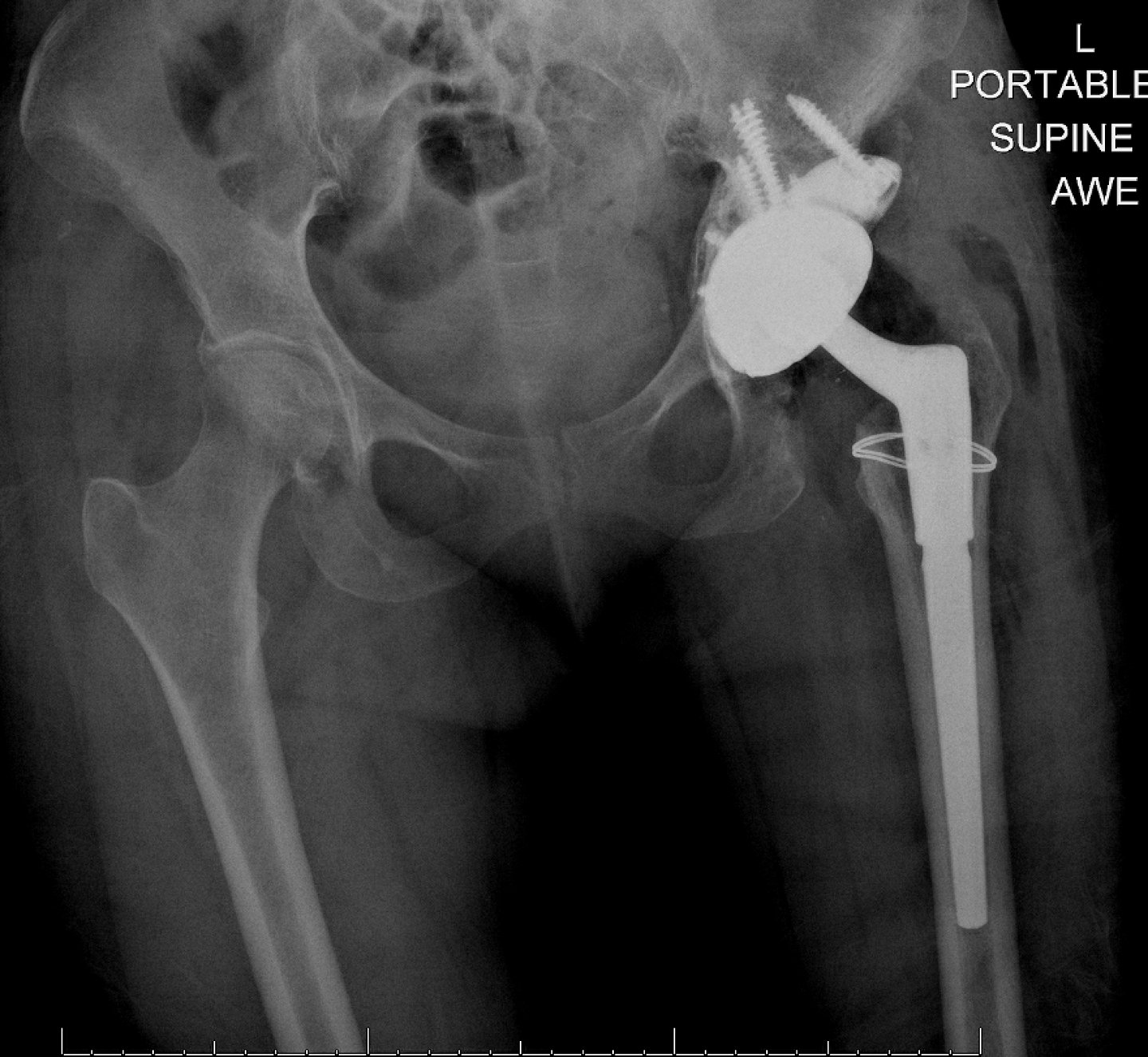
Launched in 2003, there have been over 181,000 Restoration Modular constructs implanted globally; providing solutions for femoral Type 1 through Type 4 revisions.1 This comprehensive system provides the flexibility to help address a broad range of revision scenarios with instrumentation that is designed to facilitate OR efficiency.
Key benefits
Revision Total Hip Arthroplasty (THA) should not take away from the patient’s daily activity.
Based on bench top testing, Restoration Modular has demonstrated that it can withstand daily activity after revision THA such as stair climbing and rising from a seated position.3, 5, 8, 9, 10
To enhance fatigue strength and resistance to fretting of the taper junction, Stryker Orthopaedics utilizes a proprietary shot peening process on its Restoration Modular Hip System modular taper junction.2,3
Based on bench-top testing, Stryker’s proprietary shot peening process has provided a 33% increase in taper fatigue strength compared to non-shot peened junction.5
No reported taper junction failure in over 181,000 cases since 2003.1,13
Stryker’s test parameters5:
Potting level: Distal stem is potted such that proximal / distal taper junction is above potting medium
Proximal body: 25mm +0 Cone Body
Head: +12mm Offset: 53mm
Orientation: 10° Valgus / 9° Flexion
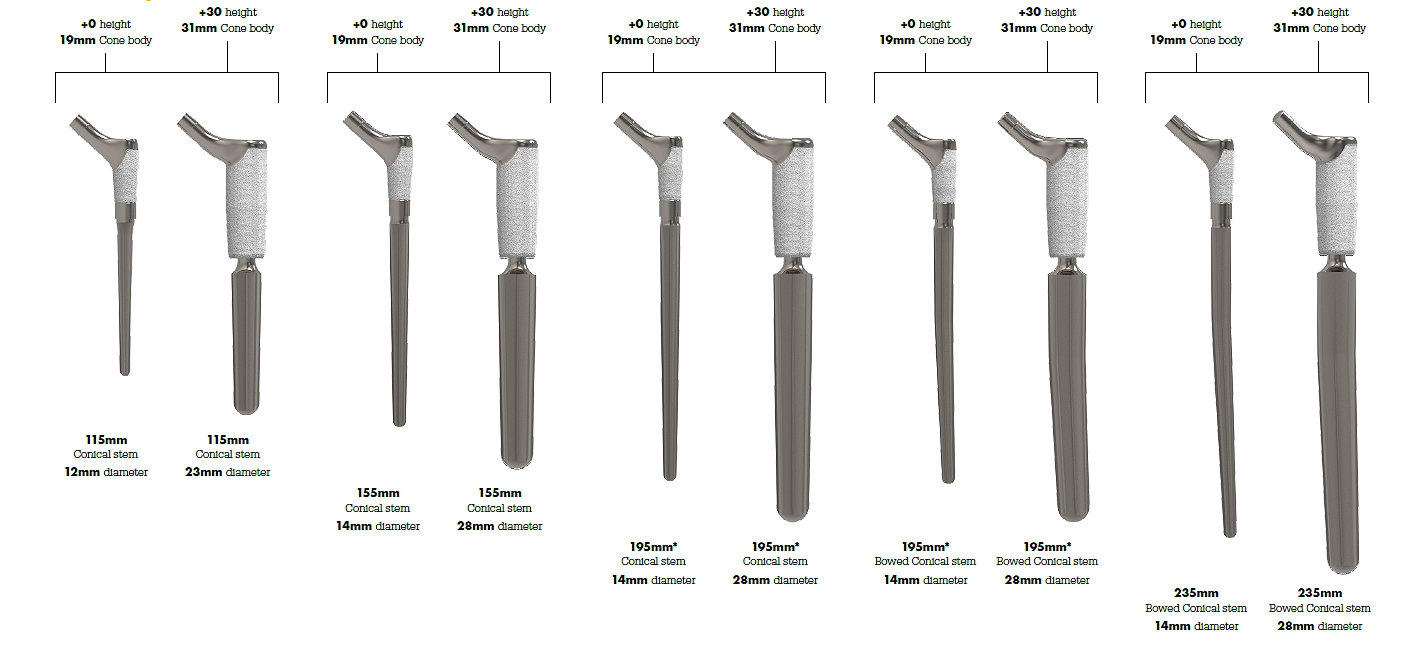
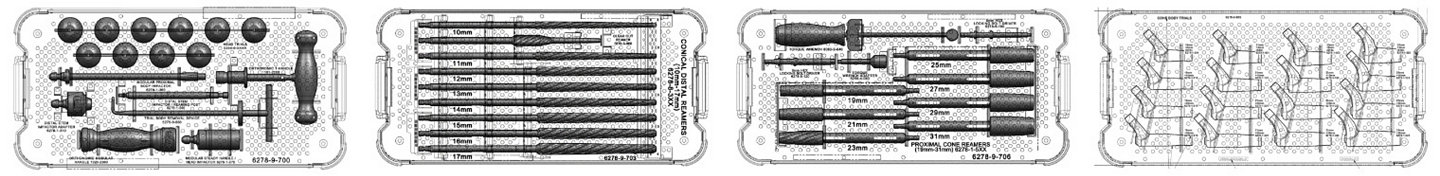
Historically simple, straight-forward instruments designed to allow for OR efficiency are further refined to fewer trays and streamlined instrumentation: helping to lower sterilization costs and creating a system more suitable for today’s healthcare environment.
Clinical evidence
Revision heritage, backed by real results“Stability was achieved in 97% of patients.”
Included Paprosky classification Type I, II, III and IV femurs.⁶
“Survivorship for the whole group was 96.9% at 5 years.”
Prospective multicenter study from 13 centers evaluating the use of the Restoration Modular System in 116 patients.⁴
“93% of patients reported being satisfied with their revision surgery.”
Retrospective review of 115 stems in 106 patients with the Restoration Modular System at a minimum follow-up of 2 years (range 2 to 9 years).⁷
Video
“Very satisfied with the new instrumentation and the short stem option. I feel as though this is a homerun. Glad to continue to use it.”
– Dr. Baratz
Milton, MA*
“LOVE IT and will plan to use Res Mod 115 for every revision case when indicated. Outstanding simplicity of instrumentation. Loved the ease of using the same instrument to insert the distal stem AND ream on top of the inserter for the proximal body. The new 115 Restoration Modular stem pairs effortlessly simple instrumentation and technique with an extremely versatile implant. This is my go-to stem for all revisions and complex primaries. The new 115 length helps me conserve bone while still offering the versatility of a modular diaphyseal fitting stem with completely customizable length, offset and version.”
– Dr. Wasterlain
Middletown, CT*
*The opinions expressed by Drs. Baratz and Wasterlain are those of Drs. Baratz and Wasterlain, respectively, and not necessarily those of Stryker. Individual experiences may vary.
DART

1. Stryker sales data 2003 - 2022
2. Shot Peening Applications. Metal Improvement Company A Subsidiary of Curtiss-Wright Corp. 9th Edition.
3. Stryker R&D Test Reprt MT05049. Restoration Modular - Median Fatigue Strength Determination of Un-Shot Peened Distal Stem Trunnions. 2005.
4. Desai RR, Malkani AL, Hitt KD, Jaffe FF, Schurman JR 2nd, Shen J. Revision total hip arthroplasty using a modular femoral implant in Paprosky type III and IV femoral bone loss. J Arthroplasty. 2012 Sep;27(8):1492-1498.e1. doi: 10.1016/j.arth.2012.03.039. Epub 2012 Jun 27.
5. Stryker R&D Test Report MT03051. Restoration Modular Taper Junction Testing (3 Stage Process). 2003
6. Restrepo C., Magdalena M., Parvizi J., Austin MS., Hozack, WJ. Clinical Orthopaedics and Related Research. Vol. 469, No. 2 February 2011: 476-482
7. Smith MA., Deakin AH., Allen D., Baines J. Journal of Arthroplasty. Vol. 31, 2016: 446-450
8. Davy DT, Kotzar GM, Brown RH, et al. Telemetric Force Measurements across the Hip after Total Arthroplasty. J Bone Joint Surg Am. 1988 January 70(1): 45-50.
9. Bergmann G, Graichen F, Rohlmann A. Is Staircase Walking a Risk for the Fixation of Hip Implants? J. Biomechanics. 1995; Vol. 28, No. 5: 535-553.
10. Stryker R&D Test Report MT02094 . Disassembly of Contaminated Ti-6Al-4V ELI Taper Specimens by an Applied Torsion. 2002.
11. Niyati Dave, Peter Tulkis, Westrich Geoffrey,MD,Ahmad Faizan. An Anthropometric Analysis Of Key Bone Morphological Parameters For A New Short Conical Stem Design. Orthopaedic Research Society 2022
12. ISO 7206-4:2010+A1:2016
13. Data on file. Stryker QMS reports. February 3, 2022.
A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery. The information presented is intended to demonstrate the breadth of Stryker's product offerings. A surgeon must always refer to the package insert, product label and/or instructions for use before using any of Stryker's products. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your sales representative if you have questions about the availability of products in your area. Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: Restoration, Stryker, Trident, Tritanium. All other trademarks are trademarks of their respective owners or holders.
Copyright © 2023 Stryker
RMOD-WC-5_34127




