Why Trust Augment?
Augment is the first and only FDA approved alternative to autograft in hindfoot and ankle arthrodesis and is backed by the largest prospective Level 1 RCT in Foot and Ankle Orthopedic history.2,*
- Proven
Level 1 evidence showing safety and efficacy in clinical outcomes when compared to the gold standard autograft in the largest F&A clinical trial ever conducted.2
- Labeled
Class III combination product specifically proven in, and labeled for, ankle and hindfoot arthrodesis via a rigorous PMA regulatory pathway.
- Unique
Bioengineered human rhPDGF-BB stimulates multiple aspects of healing in response to injury. rhPDGF-BB is highly purified with consistent biological potency; while allograft and autograft are highly variable in quality and potency.8-11
- Safe
Proven safe through multiple clinical trials and successful commercial use since 2009 in Canada and 2011 in Australia and New Zealand, while eliminating the risks, morbidities, and costs associated with autograft harvest.
*As of September, 2024
**A majority of patients studied in the two pivotal studies evaluating Augment Injectable also presented with one or more risk factors for non-union.
What is Augment
- Both Augment Bone Graft and Augment Injectable are made up of two main components, rhPDGF-BB and beta-Tricalcium Phosphate. Augment Injectable also includes a collagen matrix to make it flowable.
- A natural deficiency in PDGF-BB has been found to be correlated with non-union and poor bone formation in clinical and pre-clinical studies involving subjects with diabetes, and osteoporosis.12-14

Operative technique and MOA
Application of Augment Injectable for tibial talar fusion including mechanism of action.
What surgeons said
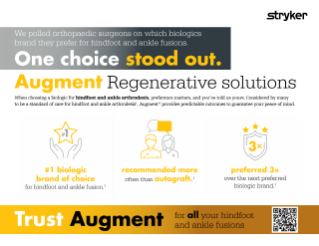
We polled orthopaedic surgeons on which biologics brand they prefer for hindfoot and ankle fusions.
One choice stood out.
Augment Regenerative solutions
When choosing a biologic for hindfoot and ankle arthrodesis, preference matters, and you’ve told us yours. Considered by many to be a standard of care for hindfoot and ankle arthrodesis1, Augment provides predictable outcomes to guarantee your peace of mind.
#1 biologic brand of choice for hindfoot and ankle fusion.1
recommended more often than autograft.1
preferred 3× over the next preferred biologic brand.1
You deserve performance and peace of mind.
Augment delivers both.
Augment is the safe, efficacious and only level 1 clinically demonstrated option for hindfoot and ankle arthrodesis, as evidenced in the largest randomized controlled trial (RCT) conducted in foot and ankle orthopaedic history. Augment is backed by Stryker’s Augment Satisfaction Guarantee.***
Reduced incidence of chronic pain.2,6
fusion success in patients over 65 (vs. autograft).3
Reduced surgical complication rate.2,4-6
More than 15 years of clinical research
Over 45 peer-reviewed publications.
Augment is the only FDA approved alternative to autograft in hindfoot and ankle arthrodesis
***Stryker’s Augment Satisfaction Guarantee provides a replacement Augment kit to a facility if a patient develops a nonunion at one or more of the ankle or hindfoot joints where Augment was implanted. The facility must meet the terms and conditions of the guarantee for the replacement to be provided.
Clinical evidence
Contact us
Send us a message to learn more about Augment
Have questions or inquiries? We’re here to help! Fill out the contact form, and our dedicated team will get back to you promptly. Your feedback and inquiries are important to us, and we look forward to assisting you with any information or support you may need.
Are you a patient looking for more information or a physician in your area?
Click the button below for our patient website.
Brief summary of important product information
Indications for use
Augment Bone Graft and Augment Injectableare indicated for use as an alternative to autograft in arthrodesis (i.e., surgical fusionprocedures) of the ankle (tibiotalar joint) and/or hindfoot (including subtalar,talonavicular, and calcaneocuboid joints, alone or in combination), due toosteoarthritis, post-traumatic arthritis, rheumatoid arthritis, psoriatic arthritis, avascular necrosis, joint instability, jointdeformity, congenital defect, or jointarthropathy in patients with preoperative or intraoperative evidence indicating the need for supplemental graft material.
Contraindications
Augment Bone Graft and Augment Injectable should not:
- be used in patients who have a known hypersensitivity to any of the components of the product or are allergic to bovine collagen (Augment Injectable only) or yeast-derived products.
- be used in patients with active cancer.
- be used in patients who are skeletally immature (<18 years of age or no radiographicevidence of closure of epiphyses).
- be used in pregnant women. The potential effects of rhPDGF-BB on the human fetus have not been evaluated.
- be implanted in patients with an active infection at the operative site.
- be used in situations where soft tissue coverage is not achievable.
- be used in patients with metabolic disorders known to adversely affect the skeleton (e.g. renal osteodystrophy or hypercalcemia), other than primary osteoporosis or diabetes.
- be used as a substitute for structural graft.
Warnings
As with all therapeutic recombinant proteins, there is a potential for immune responses to be generated to the rhPDGF-BB component of Augment Bone Graft and Augment Injectable. The immune response to rhPDGF-BB was evaluated for Augment Injectable in two studies, and for Augment Bone Graft (which contains the identical rhPDGF-BB) in two pilot and one pivotal study for ankle and hindfoot arthrodesis procedures. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Augment Bone Graft or Augment Injectable with the incidence of antibodies to other products may be misleading.
Women of childbearing potential should avoid becoming pregnant for one year following treatment with Augment Bone Graft or Augment Injectable. The implantation of rhPDGF-BB in women and the influence of their development of anti-PDGF-BB antibodies, with or without neutralizing activity, on human fetal development are not known.
The safety and effectiveness of Augment Bone Graft or Augment Injectable in nursing mothers has not been established. It is not known if rhPDGF-BB is excreted in human milk.
The safety and effectiveness of Augment Bone Graft or Augment Injectable has not been established in anatomical locations other than the ankle or hindfoot, or when combined with autologous bone or other bone grafting materials.
The safety and effectiveness of repeat applications of Augment Bone Graft or Augment Injectable have not been established. The safety and effectiveness of Augment Bone Graft or Augment Injectable in pediatric patients below the age of 18 years have not been established.
Augment Bone Graft or Augment Injectable do not have any biomechanical strength and must be used in conjunction with standard orthopedic hardware to achieve rigid fixation.
The β-TCP component is radiopaque, which must be considered when evaluating radiographs for the assessment of bridging bone. The radiopacity may also mask underlying pathological conditions. Over time, the β-TCP is intended to be resorbed at the fusion site and replaced by new bone. Under such circumstances, it would typically be indistinguishable from surrounding bone.
References:
- Double-blinded brand equity survey, GLG, 2023.
- DiGiovanni C et al, JBJS, 2013
- Berlet GC, et al. JBJSO, 2020
- Daniels TR et al, FAI, 2015
- Daniels TR et al, FAI, 2019
- Reduced chronic pain and surgical complications are related to the absence of autograft harvest
- Haddad SL, Coetzee JC, Estok R, Fahrbach K, Banel D, Nalysnyk L. Intermediate and long-term outcomes of total ankle arthroplasty and ankle arthrodesis. A systematic review of the literature. J Bone Joint Surg Am. 2007 Sep;89(9):1899-905.
- Fiedler, et al. J Cell Biochem (2002)
- Ozaki, et al. J Stem Cells & Dev (2007)
- Bouletreau, et al. Plast Reconstr Surg (2002)
- 5. Hollinger, et al. JBJS (2008)
- Verma, et al., Curr Orthop Pract (2011)
- Hollinger, et al., JOR (2008)
- Al-Zube, et al., J Orthop Res (2009)
- Carragee EJ, et al., Spine J (2011)
AP-017656