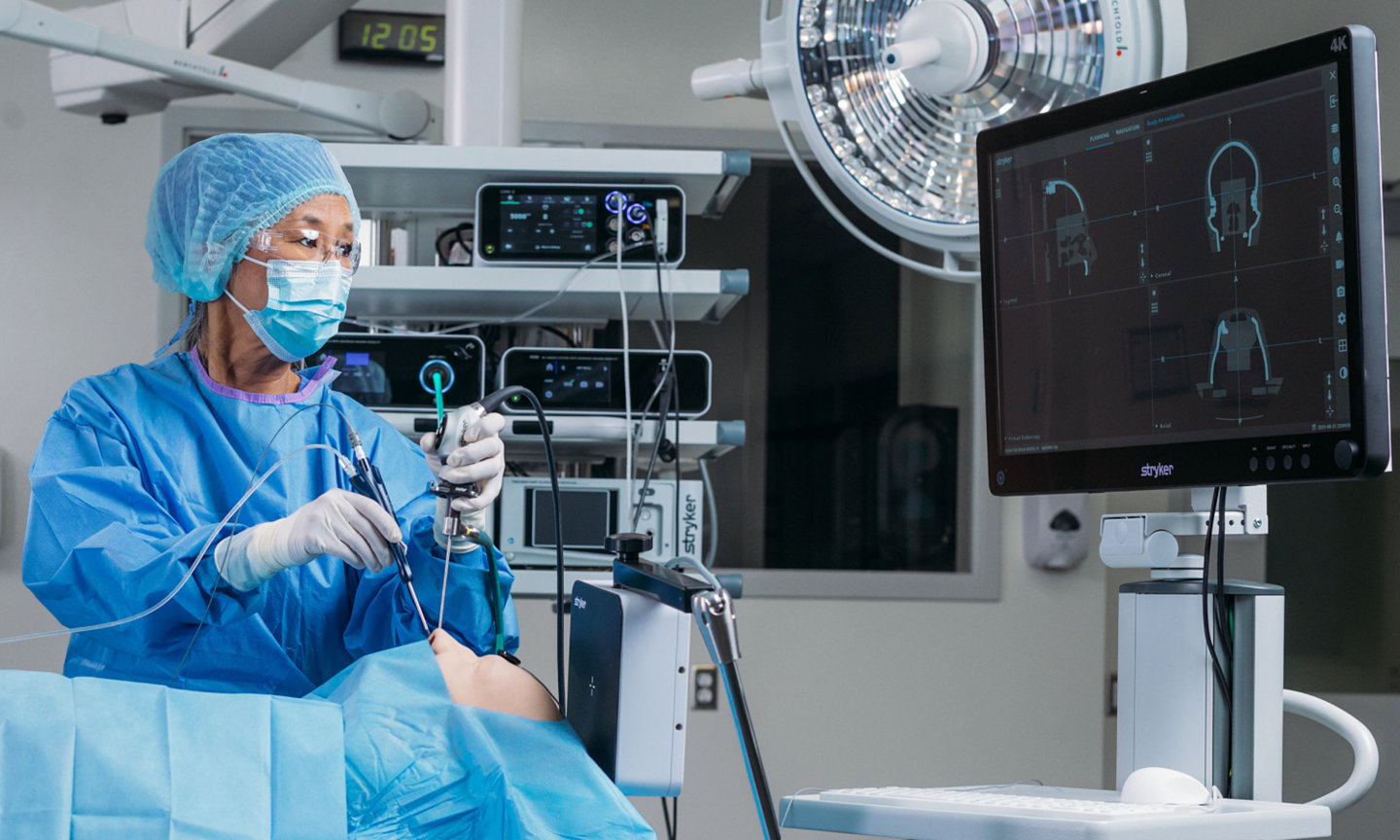
Stryker ENT navigation system
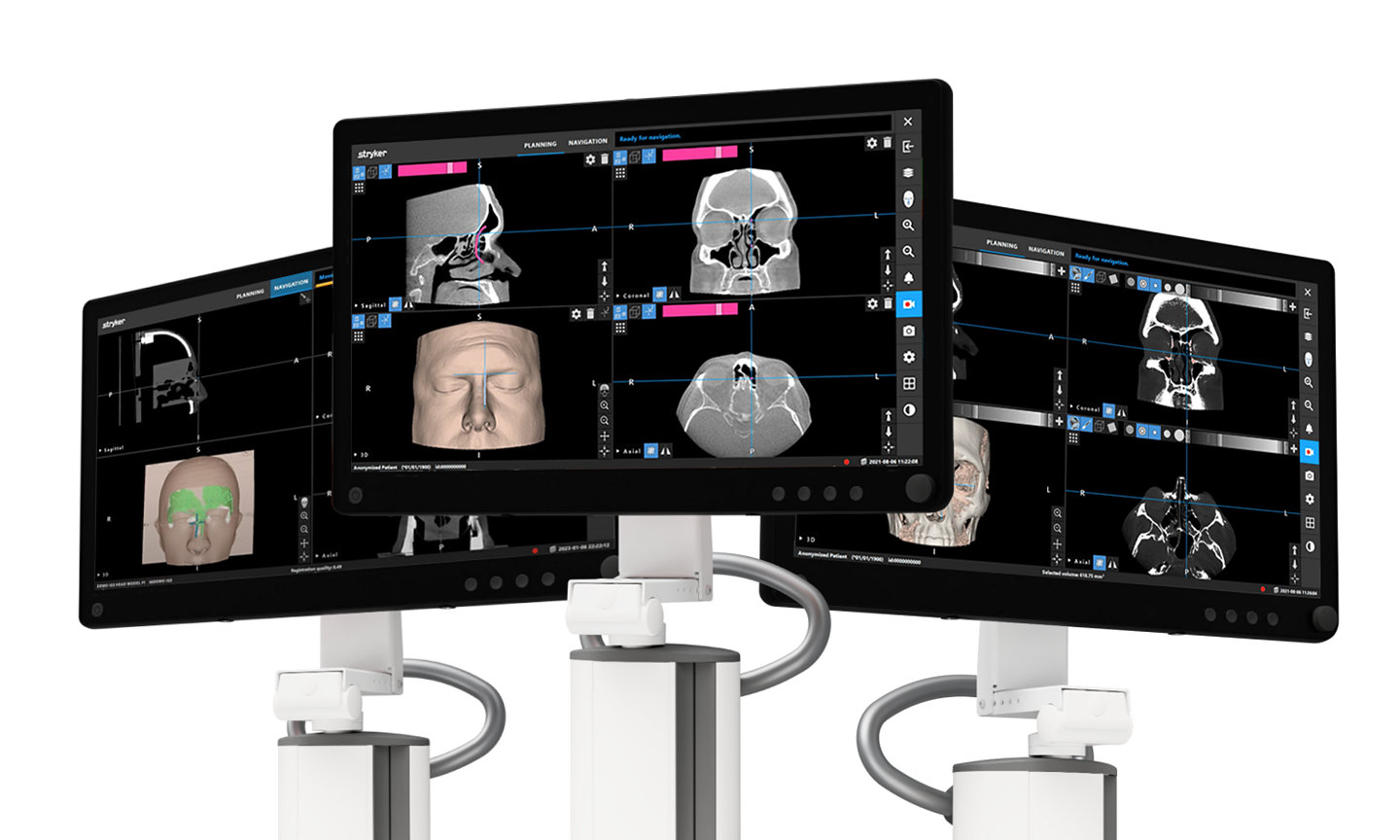
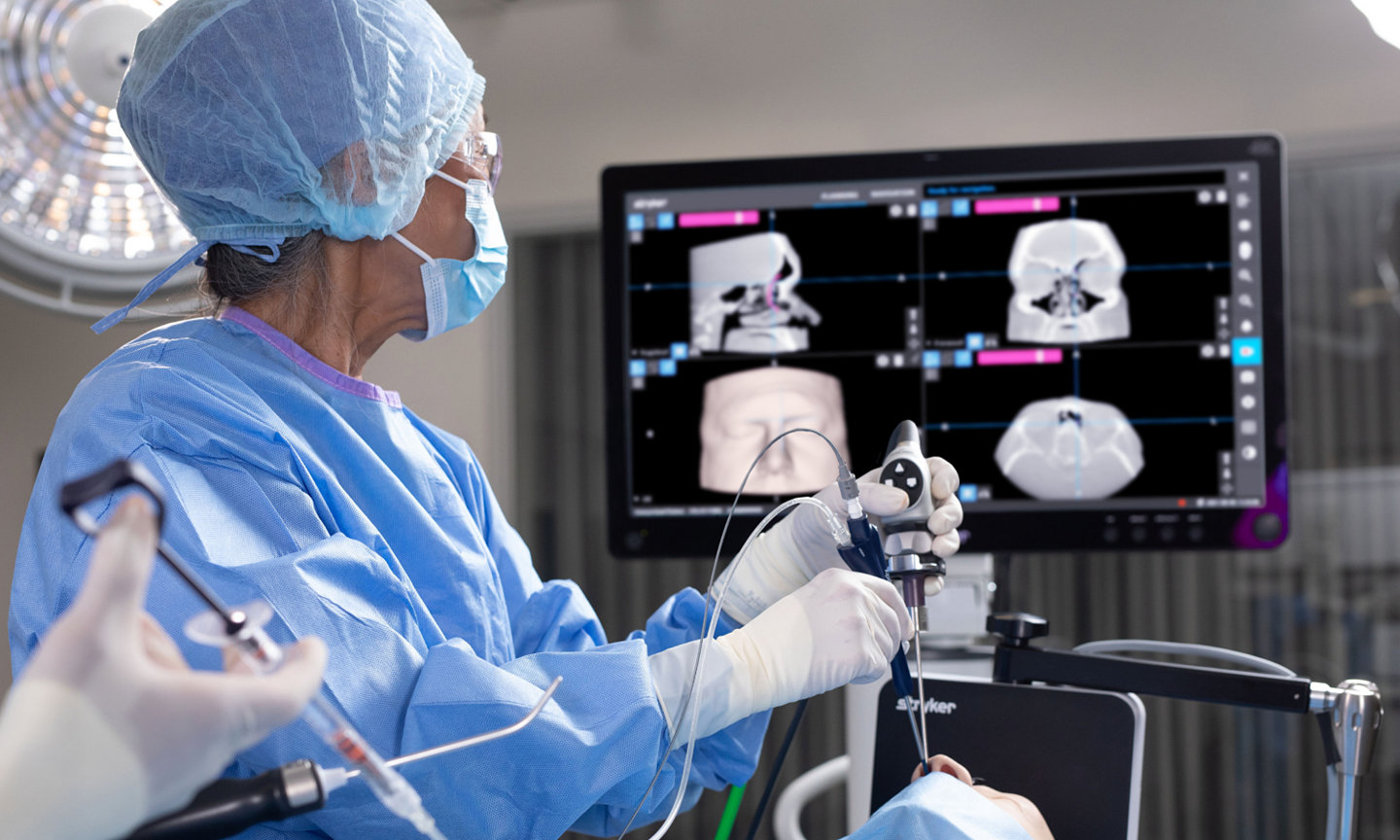
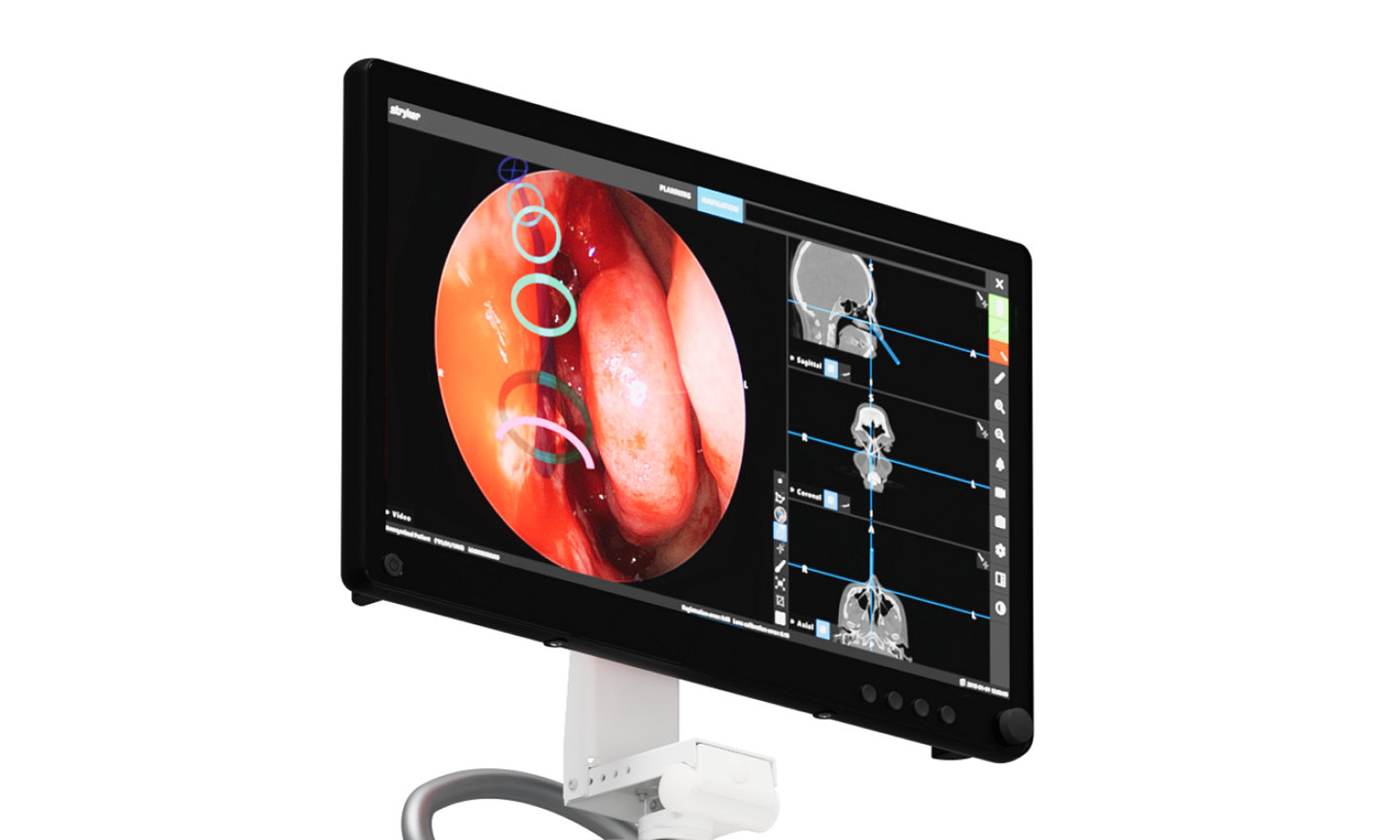
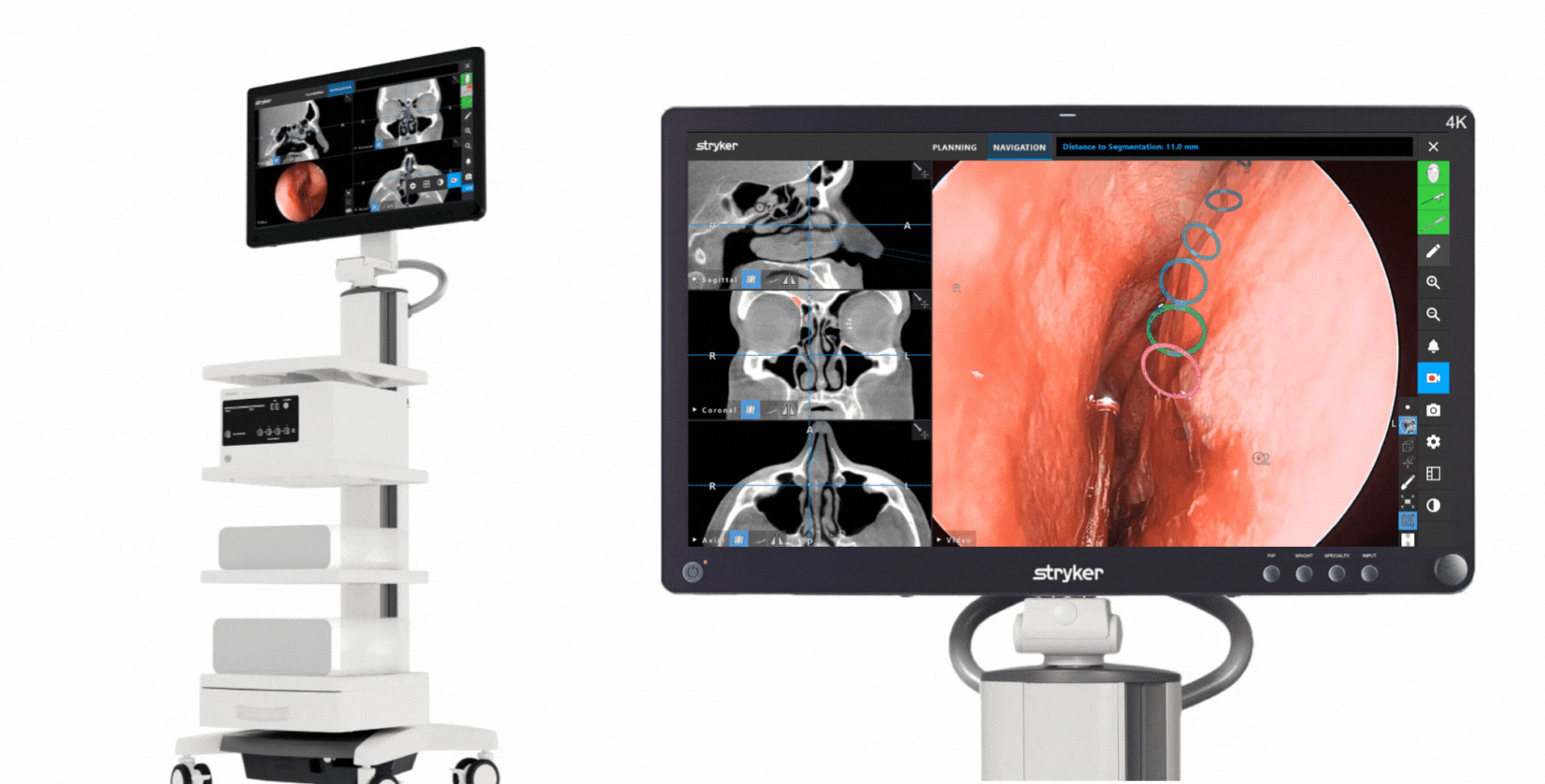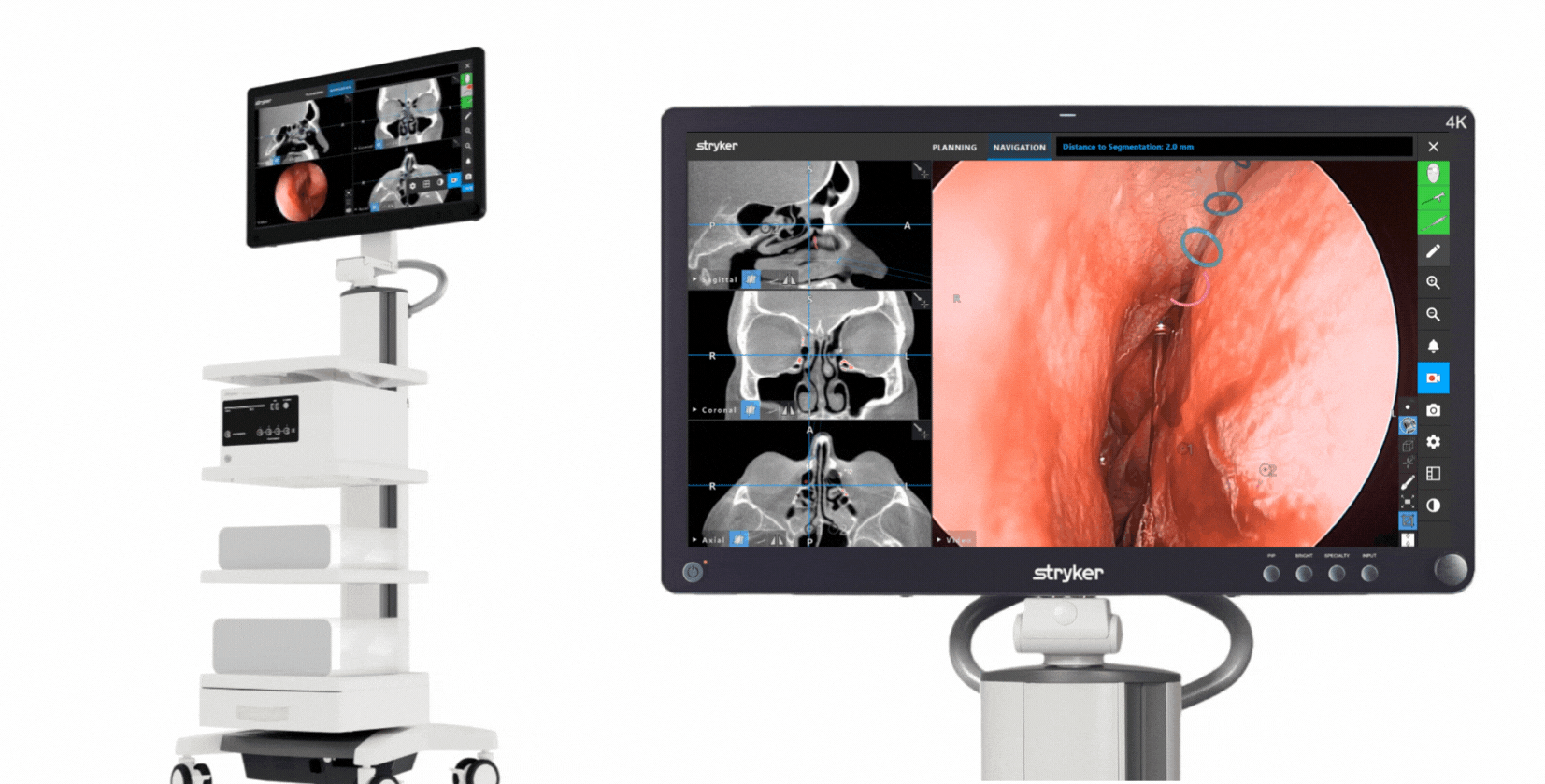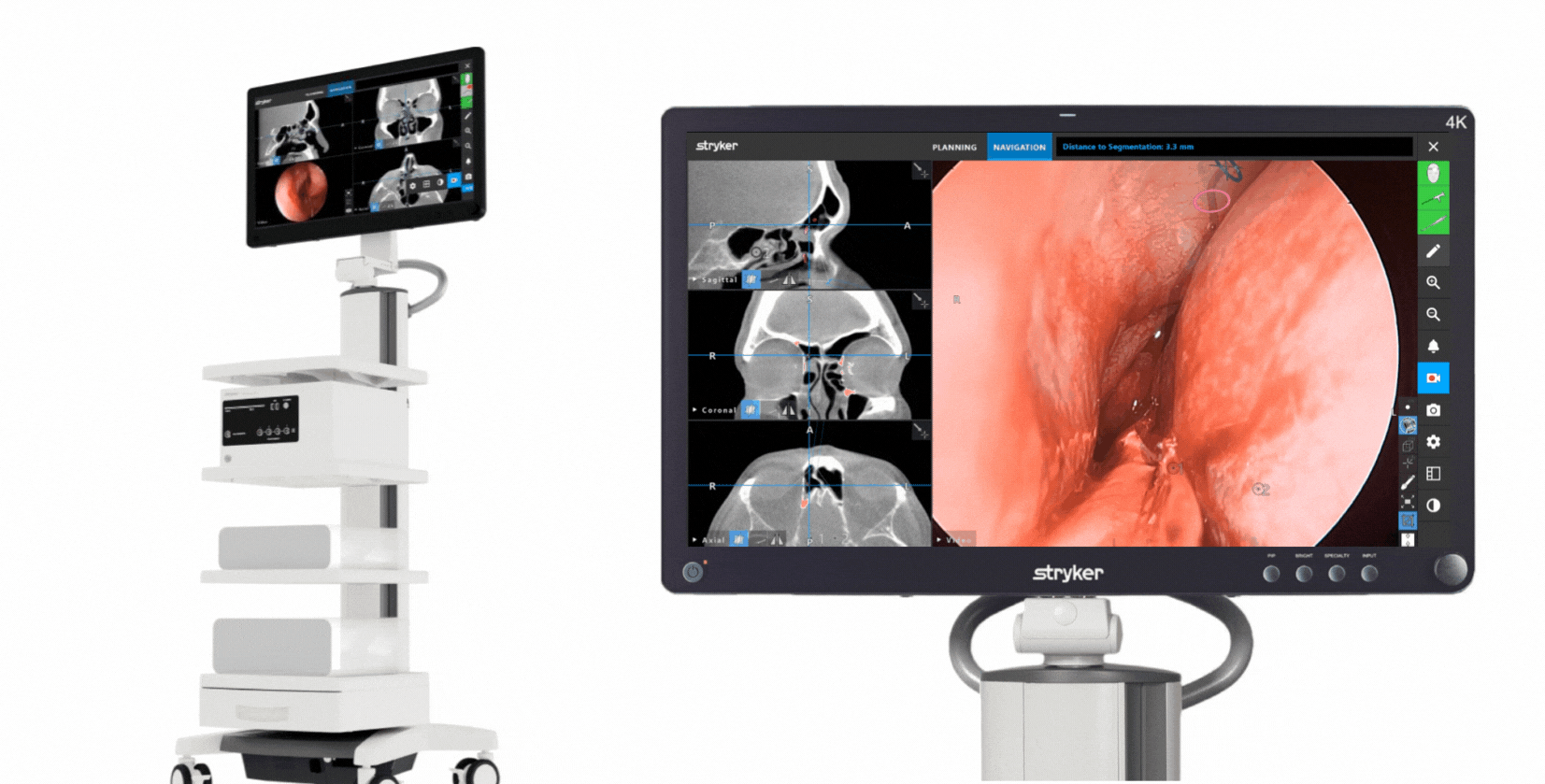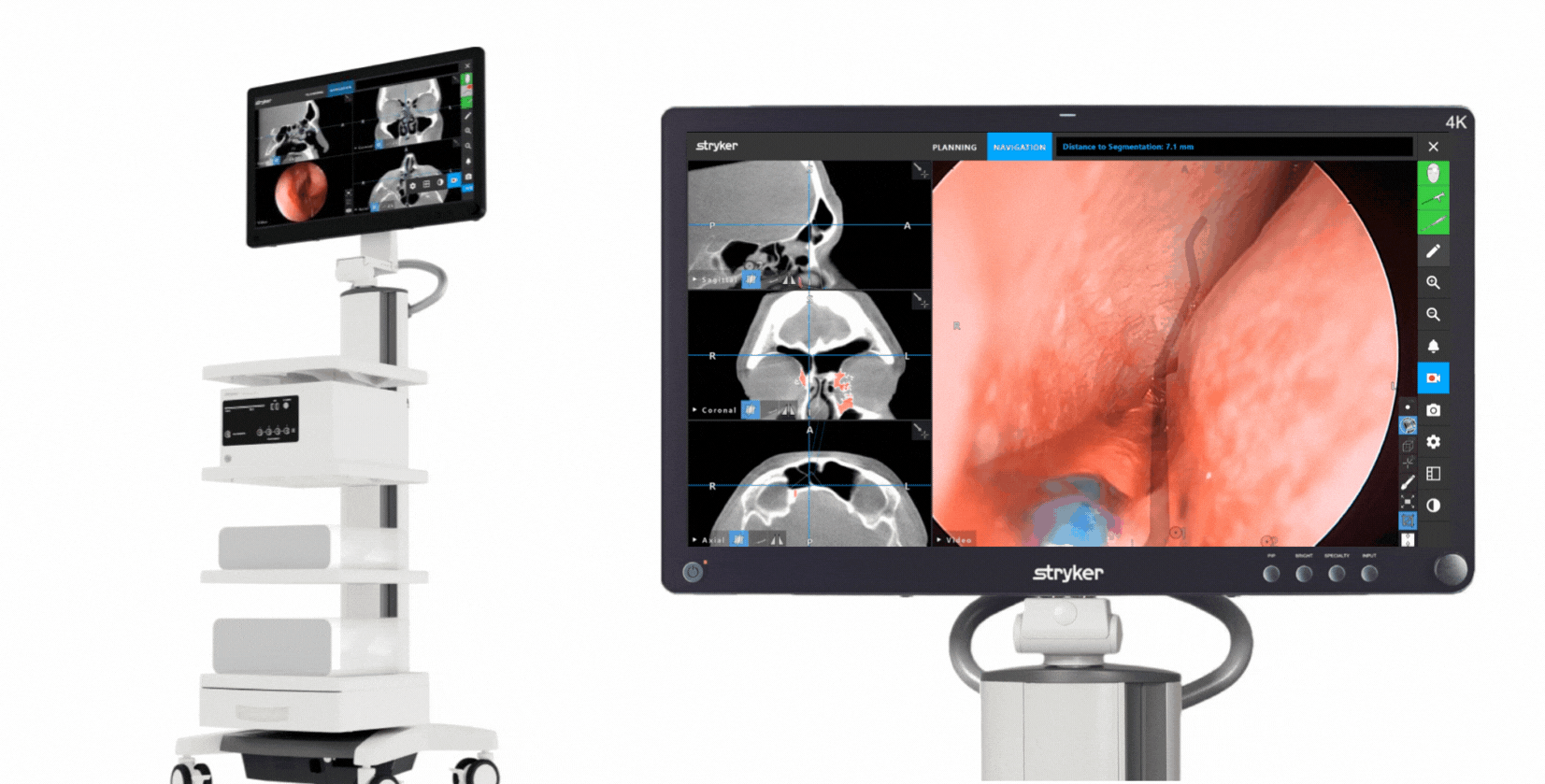
The Stryker ENT navigation system powered by Scopis software is a next-generation solution. Designed to enhance precision*, the system can make a big impact by adapting to a variety of preferences and tools while harnessing the power of planning and augmented reality leading to enhanced visibility for enhanced surgical experiences. During surgery, the unique Scopis augmented reality technology overlays planned pathways onto the endoscope image in real-time, providing you with the guidance that can help you perform a minimally invasive, accurate, and selective surgery.
Made for you to make it yours
The Stryker ENT navigation system combines groundbreaking Scopis augmented reality technology with innovative targeted-guided surgery (TGS) navigation. We’re bringing the next generation of customization capabilities to the OR. Our system is designed to adapt to your preferences, tools and workflows.
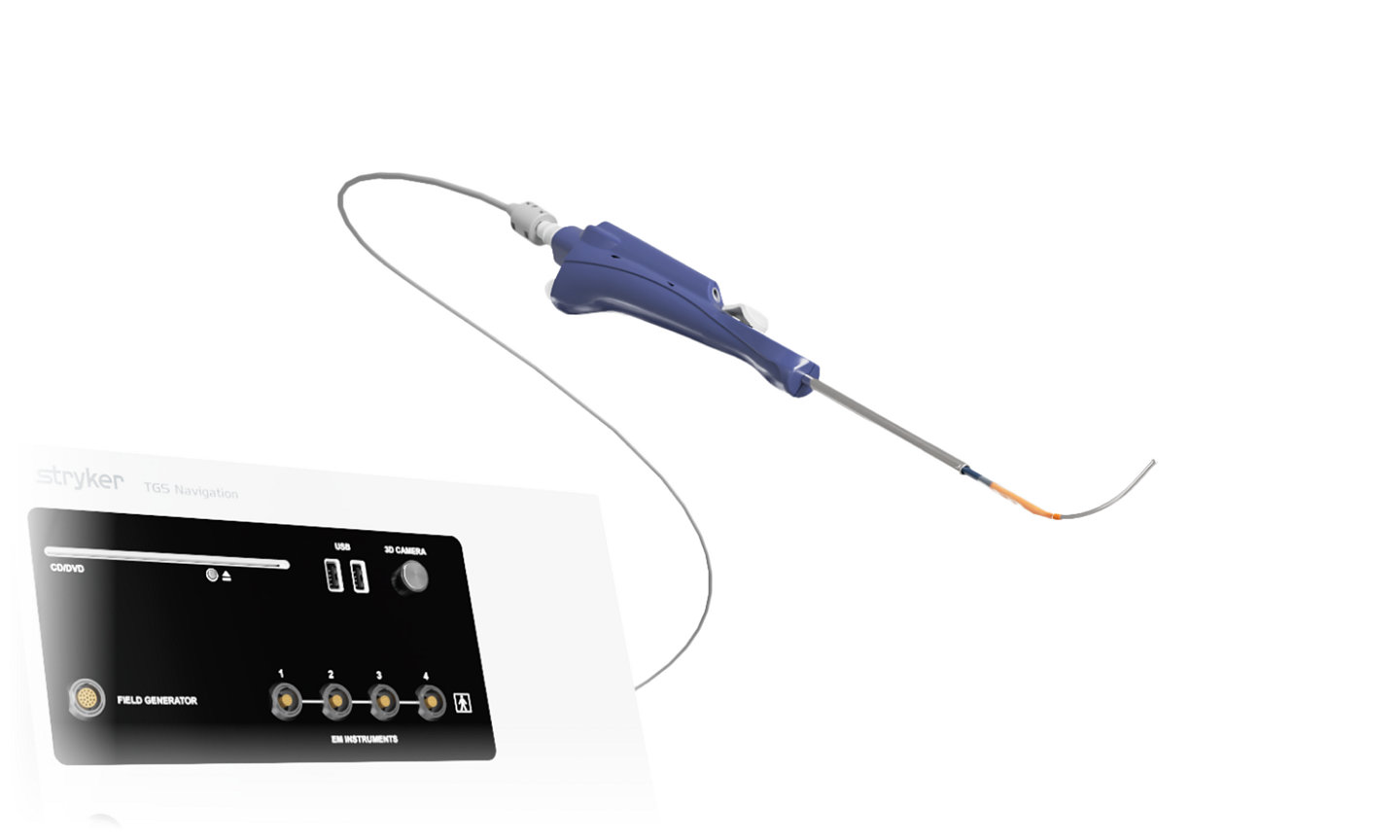
TGS guidewire and XprESS ENT dilation system
XprESS LoProfile ENT dilation system delivers a unique combination of control and versatility. The addition of the TGS guidewire enables navigation during balloon sinus dilation procedures when using the XprESS LoProfile device.
This powerful combination is designed for precision and confidence during navigation-assisted cases.*
Features and benefits
- Designed with greater control and precision in-mind during navigation-assisted procedures
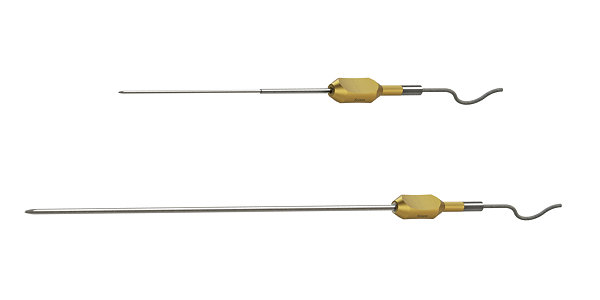
Pointers
These reusable (up to 10 times) and malleable instruments facilitate access to and precise navigation around complex anatomy.
Features and benefits
- Designed for easy access and precision during procedures
- Built-in tracking capability
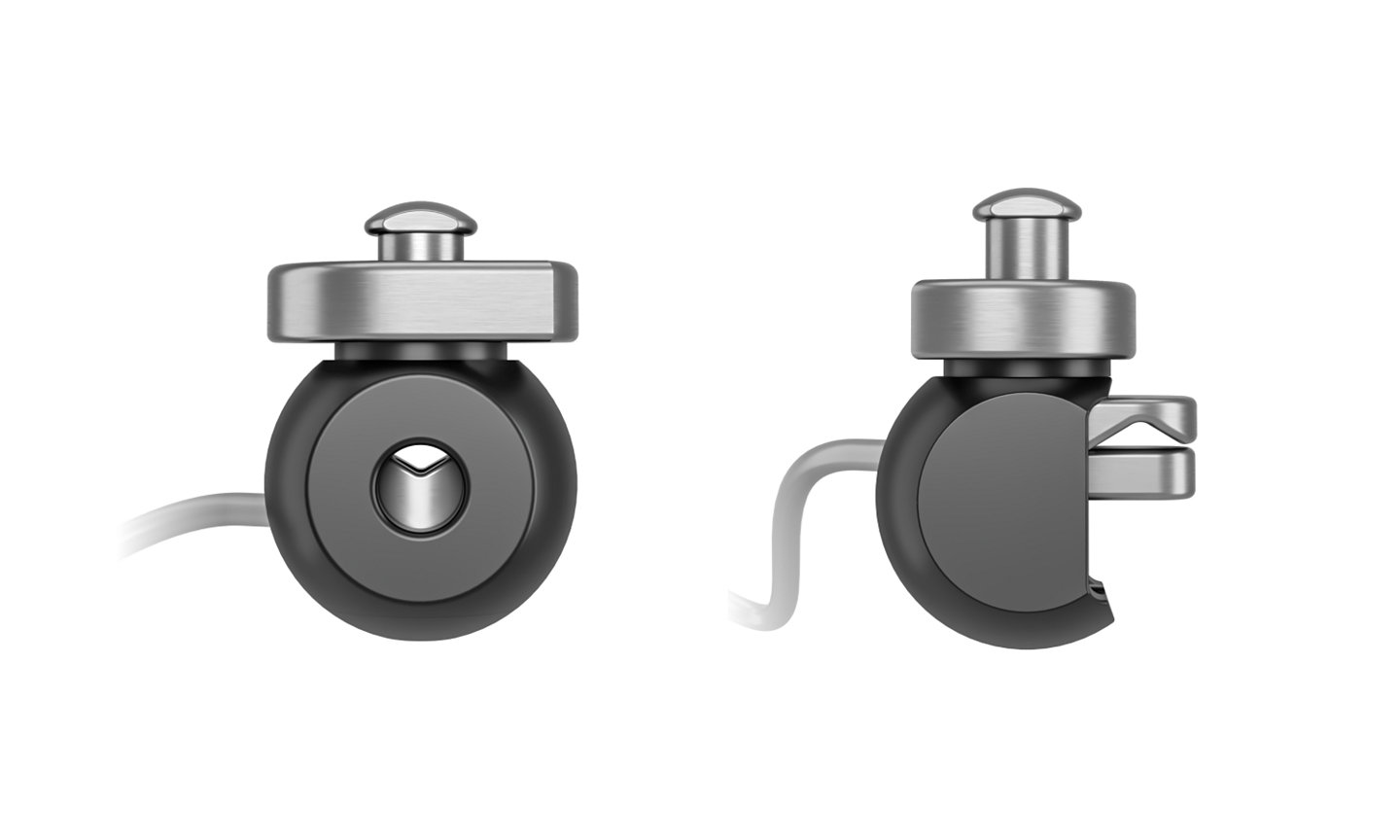
Universal instrument clamp and sphere
These instruments bring customization to new heights by adapting to your tools and workflows for a more seamless surgical experience.
Features and benefits
- Tracks nearly any rigid instrument in a matter of seconds
- Adds a low profile option for comfortability and precise navigation
| Product | Ordering no. | Description |
| Scopis ENT software | 8000-021-001 | Basic software for navigation in ENT applications |
| Scopis ENT software with TGS (Target Guided Surgery) technology | 8000-021-002 | Premium software for planning and navigation in ENT applications with advanced planning features, virtual endoscopy and augmented reality technology |
| Electromagnetic navigation unit | 8000-010-003 | High-performance navigation PC with electromagnetic measuring technology |
| Field generator | 8000-010-004 | Accessory used for the electromagnetic position measurement of patient trackers and navigated instruments |
| Field generator mounting arm | 8000-010-005 | Flexible mounting arm to position the field generator |
| Equipment cart pro | 8000-030-002 | Includes four shelves and a storage drawer |
| Medical keyboard | 8000-030-010 | U.S./international keyboard, wired, number pad, USB |
| Mouse, wired | 8000-030-020 | Wired, USB, three buttons, scroll function |
| Patient tracker tabs | 8000-100-001 | Adhesive pads for fixation of patient trackers electromagnetic, 100 per unit |
| Patient tracker electromagnetic mini disinfectable red hub | 8000-040-001 | Disinfectable, used to locate the patient within the EM field |
| Registration pointer electromagnetic | 8000-050-003 | Disinfectable, used for surface registration |
| Navigation tool extension cable | 8000-050-011 | Extension cable for navigation tools |
| Instrument clamp, forceps | 8000-060-010 | For attaching the universal tracker to rigid conventional surgical instruments |
| Instrument clamp, 2-6 mm | 8000-060-011 | For attaching the universal tracker to rigid conventional surgical instruments between 2-6 mm |
| Instrument clamp, 6-10 mm | 8000-060-012 | For attaching the universal tracker to rigid conventional surgical instruments between 6-10 mm |
| Instrument clamp, 10-16 mm | 8000-060-013 | For attaching the universal tracker to rigid conventional surgical instruments between 10-16 mm |
| TGS guidewire (single use) | 8000-060-009 | Allows navigation of XprESS LoProfile system |
| Patient tracker electromagnetic (maximum 10 uses) | 8000-040-002 | Used to locate the patient within the EM field |
| Precision pointer electromagnetic (maximum 10 uses) | 8000-050-001 | 1.5 mm, malleable |
| Suction tube, Frazier electromagnetic (maximum 10 uses) | 8000-050-005 | 3 mm, malleable, straight suction |
| Suction tube, Eicken electromagnetic (maximum 10 uses) | 8000-050-006 | 3 mm, malleable, curved suction |
| Endoscope tracker electromagnetic (maximum 10 uses) | 8000-060-001 | Navigation suitable for 0°, 30° and 45° endoscopes with 4 mm diameter and 180 mm length |
| Calibration body electromagnetic (maximum 10 uses) | 8000-060-002 | Calibration of suitable conventional surgical instruments |
| Universal tracker electromagnetic (maximum 10 uses) | 8000-060-006 | Allows navigation of rigid conventional surgical instruments in combination with an instrument clamp |
| Instrument clamp sphere (maximum 10 uses) | 8000-060-020 | Adapter to track rigid conventional surgical instruments between 2-4.6 mm |
| Instrument clamp universal (maximum 10 uses) | 8000-060-021 | 3-6 mm, universal tracker |
Important risk and safety information
To safely use our ENT navigation system, review the Indications for Use.
Interested in learning more about the Stryker ENT navigation system? Contact your rep and set up a trial.
*Designed to locate anatomical landmark of interest within 2 mm
**The TGS guidewire enables navigation during balloon sinus procedures when using the XprESS LoProfile device.
References
Data on file at Stryker D0000013273(UN606; SR773)
Citardi MJ, Agbetoba A, Bigcas JL, Luong A. Augmented reality for endoscopic sinus surgery with surgical navigation: a cadaver study. Int Forum Allergy Rhinol. 2016;6: 523–528
Stryker ENT Navigation system, instructions for use. D0000009107
Stryker ENT software 3.6.0, instructions for use. D0000060936
Stryker ENT software 3.4.54, instructions for use. TD8000010704
Field Generator, instructions for use. TD8000010701
Patient Tracker Electromagnetic, instructions for use. TD8000040700
Patient Tracker Tabs, instructions for use. TD8000100700
Registration Pointer, instructions for use. TD8000050701
Precision Pointer, instructions for use. TD8000050700
Suction Tube Fraizer Electromagnetic and Suction Tube Eicken Electromagnetic, instructions for use. TD8000050702
Instrument Clamp Electromagnetic Sphere and Instrument Clamp Electromagnetic Universal, instructions for use. 700000842818
TGS Guidewire, instructions for use. 5176-001
Field Generator Mounting Arm, instructions for use. TD8000010702
XprESS ENT Dilation System, instructions for use. 4322-001
- Scopis ENT software CT/MRI imaging protocol. 700001231992
This document is intended solely for the use of healthcare professionals. A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. We do not dispense medical advice and recommend that surgeons be trained in the use of any particular product before using it in surgery.
The information presented is intended to demonstrate Stryker’s products. A surgeon must always refer to the package insert, product label and/or instructions for use, including the instructions for cleaning and sterilization (if applicable), before using any of Stryker’s products. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your representative if you have questions about the availability of Stryker’s products in your area.
Stryker or its affiliated entities own, use, or have applied for the following trademarks or service marks: TGS, Scopis, Stryker and XprESS. All other trademarks are trademarks of their respective owners or holders. The absence of a product, feature, or service name, or logo from this list does not constitute a waiver of Stryker’s trademark or other intellectual property rights concerning that name or logo.
ENT-NAV-SYK-665390