LATERA® absorbable nasal implant system
LATERA supports upper and lower lateral nasal cartilage, which may reduce nasal airway obstruction (NAO) symptoms and help increase nasal airflow.1
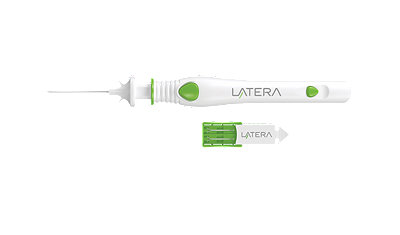
Features and benefits
- The forked tip helps securely anchor the implant in place9-10
- Implants are available in 20mm and 24mm lengths and are made of absorbable PLLA polymer9-10
- The atraumatic ball tip is designed to minimize tissue damage9-10
Related categories
How is the LATERA implant absorbed?
The LATERA implant is absorbed over a period of approximately 18-24 months. Post implantation, a fibrous capsule forms and the integrity of the implant is maintained through 12 months. Tissue encapsulation promotes acute implant stability and enables localized tissue response during the absorption process. Remodeling occurs once the implant is replaced with fibrous collagen construct to provide ongoing support.2
- Implantation: Implant integrity maintained while fibrous capsule forms.2
- Encapsulation: Tissue encapsulation promotes acute implant stability. Safety profile shows minimal inflammatory response and expected surrounding tissue reaction.2
- Remodeling: Capsule and fibrotic tissue remains as implant degrades and is absorbed. Collagen construct replaces implant at approximately 24 months.2
Watch how the LATERA implant works
The LATERA implant is inserted with a 16-gauge cannula delivery device and supports the upper and lower lateral cartilage by anchoring above the maxilla to provide cantilever support.
Product specifications
| Product | Ordering no. | Description |
| LATERA absorbable nasal implant system | LATSYS20 | 20 mm, 1 system |
| LATERA absorbable nasal implant system | LATSYS24 | 24 mm, 1 system |
1 system includes: 1 implant delivery device, 2 absorbable nasal implants
Patient results
Patients treated with the Latera absorbable nasal implant showed significant improvement in symptoms when measured with the nasal obstruction symptom evaluation (NOSE) score.3
While individual results may vary, patients treated with LATERA absorbable nasal implant experienced the following symptom improvements:1
- Reduced nasal congestion or stuffiness
- Less trouble breathing through the nose
- Improved nasal breathing during exercise or exertion
- Reduced nasal blockage or obstruction
- Less trouble sleeping
Will my patient’s appearance change?
Patients experienced a reduction in nasal obstruction symptoms of 57.7% at two years, as measured by the NOSE survey. Patients achieved these results without negative cosmetic effects.6
Risks included temporary symptoms such as:7
- Mild bruising and inflammation
- Awareness of the implant
- Mild pain or irritation
Other risks related to the LATERA implant included: discomfort, infection, reaction to material and surgical implant removal.9
In a clinical study, patients experienced no long-term adverse cosmetic changes with the LATERA implant.1
of patients* were satisfied with their breathing after the procedure8
of patients* were satisfied with their appearance after the procedure8
* Individual patient results may vary and may include other procedures. Use of the LATERA device in conjunction with other procedures (such as septoplasty and/or turbinate reduction) has not been clinically evaluated. Patient satisfaction results may be attributed to LATERA with other procedures.
What patients have experienced with LATERA
Interested in learning more about Latera? Contact your rep and set up a trial.
REFERENCES
- San Nicolo M,et. al. 2017. Absorbable Implant to Treat Nasal Valve Collapse. Facial Plast Surg, 32:233-240.
- Rippy MK, Baron S, Rosenthal M, Senior BA. Evaluation of absorbable PLA nasal implants in an ovine model. Laryngoscope Investig Otolaryngol. 2018; 3(3):156-161
- Chandra RK, Patadia MO, Raviv J. Diagnosis of nasal airway obstruction. Otolaryngol Clin North Am. 2009 ;42(2):207-25.
- American Academy of Otolaryngology -- Head and Neck Surgery. (2010, January 4).
- ScienceDaily. Retrieved July 31, 2022 from www.sciencedaily.com/releases/2010/01/100101011826.htm
- San Nicoló M, Stelter K, Sadick H, Bas M, Berghaus A. A 2-Year Follow-up Study of an Absorbable Implant to Treat Nasal Valve Collapse. Facial Plast Surg 2018; 10.1055/s-0038-1672213.
- Stolovitzky, et. al. 2018. A Prospective Study for Treatment of Nasal Valve Collapse Due to Lateral Wall Insufficiency: Outcomes Using a Bioabsorbable Implant. Laryngoscope.
- Data on file TR-21076 Spirox NVC Experience.
- LATERA Instructions for Use 4645-002
- Stryker Data on File DC-0000002742
ENT-LATSYS-SYK-665351
Last Updated April/2024

