Vocera Smartbadge
Enhanced hands-free communication
Simplify hospital communication and workflow to help improve staff safety and impact on patient care.


Streamline hospital communications
Care teams face mounting challenges daily and need a solution that streamlines communication, minimizes interruption fatigue and safeguards staff - without comprimising patient care.
The Smartbadge can help by:
- making it easy for people to find each other and communicate quickly
- enabling people to communicate safely and effectively during hands-on patient care workflows
- assisting workers to reach help quickly in an emergency
Purpose-built for patient care
- Communicate intuitively: Make, receive, transfer and forward calls using a natural, voice-driven experience. Say, “OK Vocera” to wake up the device and start communicating.
- Improve staff safety: Communicate safely, even under restrictive personal protective equipment (PPE), to help reduce the spread of infection. In an emergency, activate the dedicated panic button to discreetly open a communication channel to the response team.
- Help reduce interruption fatigue and stay focused on critical patient care: Set your device to do-not-disturb mode and accept or decline notifications. Avoid communication dead ends with call escalation to the next available person or team.
- Easily reach the right person or team: Reach contacts by saying their name, role, group or extension. Page a user or group. Call people inside or outside your health system.
- Triage events quickly: Determine the urgency of events via notifications with detailed context on the Smartbadge.
- Leverage smartphone functionality: Get smartphone usability and hands-free mobility with the Smartbadge’s ample touchscreen.

Clear communication
- Enjoy smooth, natural bidirectional conversation
- Send and receive secure messages as easily as on a smartphone
- Make and answer calls hands-free
- Communicate safely, even under restrictive PPE
Simple functionality
- Combine smartphone usability and hands-free mobility
- Ample touchscreen to find contacts quickly, with a near-real time view of patients and staff across multiple sites
- Read and type text messages, and receive notifications with context about the patient, event and care team

Stay in the know
- Enable near-real time situational awareness and help reduce interruption fatigue
- Receive voice calls, messages and notifications with essential information available at-a-glance
- Quickly connect from anywhere with the right person simply by saying the name, role or group name of whom you want to reach
Vocera Smartbadge's key features

Hands free calling
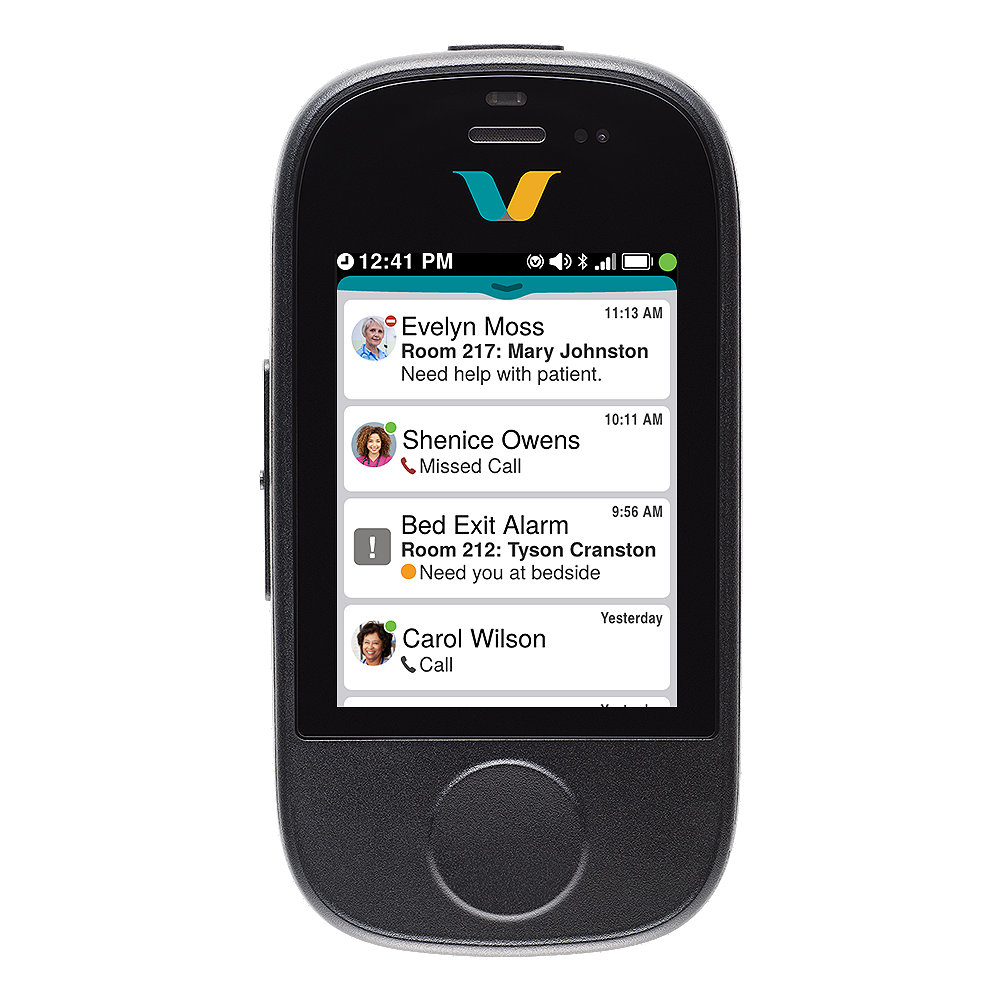
Unified Inbox
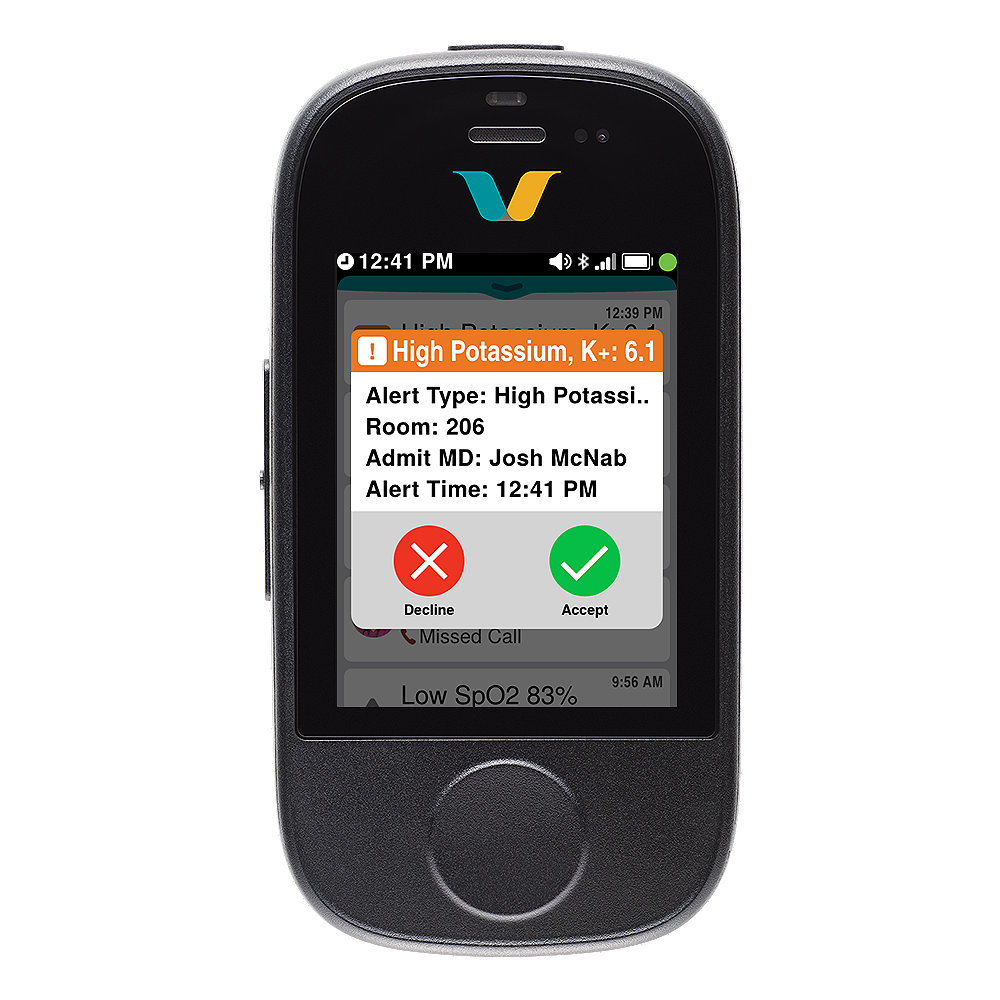
Alarms with context*
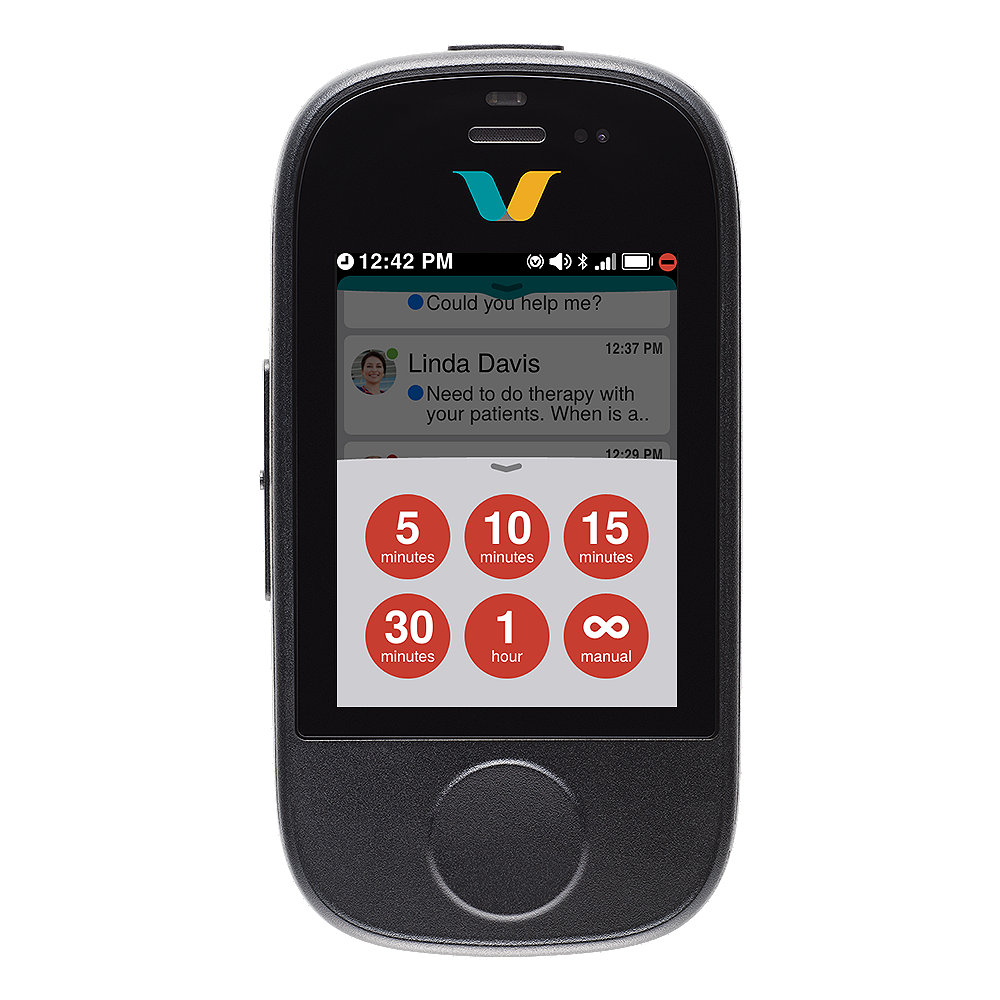
Do not disturb
Device features
| 2.4" touchscreen with haptic touch call button |
| 89g |
| 100mm x 52mm x 17mm |
| Swappable battery with 4hrs active life, 40 hrs standby |
| Dedicated panic button |
| Ruggedized for healthcare with IP54 Ingress Protection |
| Bluetooth-enabled |
| Dedicated handset receiver |
| USB-C charging port |
Core functions
| Place and receive calls |
| "OK Vocera" to awaken device |
| Send / receive secure messages (No character limit) |
| Receive intuitive notifications with additional context* |
| Search users and groups on screen, and view presence/availability |

Get even more from the Smartbadge with workflow intelligence
Help reduce alarm fatigue and make it easier for clinicians to stay focused on patient care by coupling Vocera hands-free communication devices with the Vocera Engage intelligent workflow engine. Engage enables integration with more than 150 clinical and operational systems. It allows routing, escalation and prioritization of communications and notifications that include patient, event and care team context.
With Engage you can:
- Receive prioritized messages and alarm notifications based on rules set by your organization
- Listen and respond to alarm notifications hands-free or by using the Call and DND buttons
- Add yourself to a group
Interested in learning more? Talk to a rep today.
Training and education
Vocera legal
Professional services
Vocera Accessories
Related products
Explore Vocera products and solutions.
Vocera Engage
Vocera Engage middleware is the core of Vocera’s communication and workflow intelligence. It provides care teams a more complete picture of a patient’s situation, enabling prompt communication and helping to enhance patient safety.
Learn moreVocera Edge
Help enhance care team mobility and simplify the work at the point of care through effective, reliable clinical workflows and communication.
Learn moreVocera Minibadge
Hands-free communication for complex hospital environments. Triage urgency of events, call for help and more without needing to use your hands.
Learn moreSync Badge
Inspired by nurses and designed for fast, reliable collaboration and enhanced workflow efficiency
Learn more*Alarm notifications require Engage Medical Device Alarm Notification (EMDAN), FDA 510(k)-cleared middleware.
M0000015114 REV AA







