Tornier Perform
Reversed Glenoid & Reversed Augmented Glenoid
Designed for control in reverse shoulder arthroplasty cases.
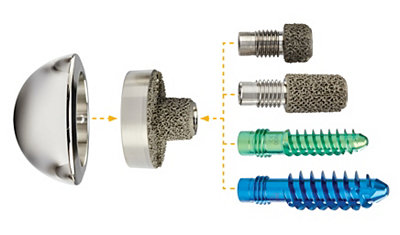
Tornier Perform Reversed Glenoid was developed to address cuff tear arthropathy and osteoarthritis, and designed to address all glenoid bone loss classifications from Walch and Sirveaux.
Related categories
Key benefits
Fixation
- Initial stability with modular 6.5/9.5 central screw. Long-term stability with Adaptis Integrated Porous Metal
Augmentation
- Defect-mimicking implants designed for bone preservation.
Orientation
- Control through infinite dial-able placement.
Key benefits
- The Tornier Perform Reversed Augmented Glenoid features four augmented baseplates (25 mm Half-Wedge or Full-Wedge, and 29 mm Half-Wedge or Full-Wedge).
- The wedged portion of the baseplates utilise Stryker's Adaptis Integrated Porous Metal and was designed to encourage bone ingrowth and may assist in fixation strength.

AP-015746


