Stryker’s Exeter hip stem turns 50
24-Nov-2020
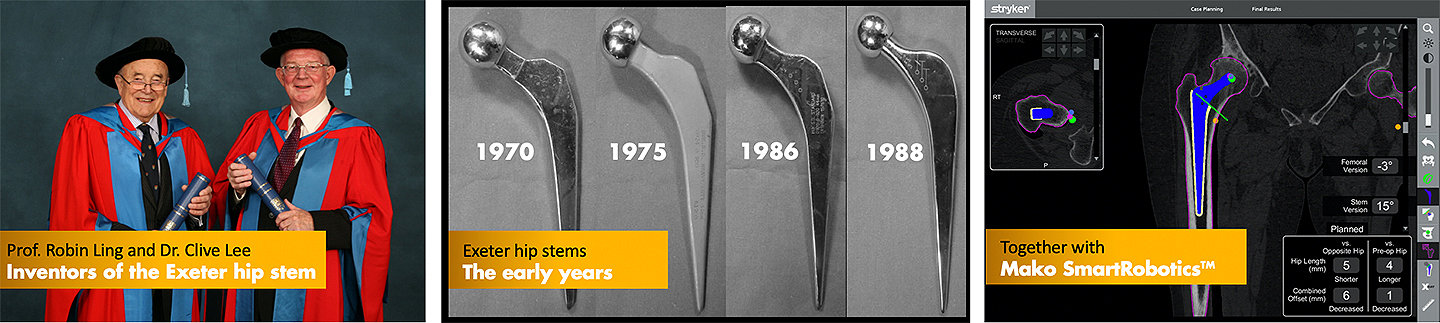
On Friday, the 27th of November 2020, Stryker marks the 50th anniversary of the Exeter cemented hip stem, an implant developed in collaboration between Prof. Robin Ling, a surgeon of the Princess Elizabeth Orthopaedic Hospital, and Dr Clive Lee, an engineer of the University of Exeter, to address the problem of mechanical loosening.
Developed in 1970, Exeter was the first collarless, polished and tapered hip stem, a design that helped to significantly reduce implant loosening and the rate of revision surgery.1,5 Prof. Ling and Dr Lee realized that many revision surgeries were performed due to loosening of the hip implants on the market at the time. There was a need to design a stem that was well fixed and could also be inserted through a surgical approach requiring only one assistant. To attempt to solve these problems, Prof. Ling and Dr Lee partnered with a medical device maker to develop a new implant.
Over the last 50 years, Exeter has evolved to enable leading-edge implant design and surgical techniques, constantly evolving to enable surgeons to intraoperatively restore a wide variety of patient anatomies and help their patients achieve “a forgotten hip.” In 2016, the first Mako SmartRoboticsTM case using an Exeter hip stem was performed by Prof. Ross Crawford in Brisbane, Australia. Today, Exeter remains the only cemented stem available for robotic total hip arthroplasty.
“The Exeter stem is implanted in countries all over the world. National joint registries have confirmed excellent results and use of the Exeter hip continues to grow on all continents,” said Prof. John Timperley, Consultant Orthopaedic Surgeon of the Princess Elizabeth Orthopaedic Centre and Nuffield Health Hospital, Exeter. "This hip system has stood the test of time. It continues to deliver impressive clinical outcomes because the surgical team, in collaboration with Stryker, continues to evolve and refine techniques, including use of the stem with Stryker’s Mako Technology."
Compared to industry standards, the Exeter hip stem continues to be a leading choice of implant on several national joint registries around the world across all patient age groups. In a 2016 study,2 long-term survivorship for aseptic loosening was 99% at 22.8 years follow-up in a diverse patient group. In a 2020 study,3 patients 50 years or younger implanted with an Exeter stem demonstrated 96.3% survivorship for aseptic loosening.
Not only has the Exeter Hip Unit at the Royal Devon and Exeter NHS Trust reported excellent long-term results from their own centre, other surgeons and institutions have also appreciated strong and consistent outcomes. A study4 published earlier this year compared the U.K. National Joint Registry data with results from the Exeter Hip Unit. The study found that the results from the Exeter Hip Unit were far superior compared with the national average; improved results were associated with the use of the Exeter hip stem.
"It’s incredibly rare to see an implant, or really any medical device, that has stood the test of time like Exeter,” said Kathy Truppi, vice president of marketing for Stryker’s Joint Replacement division. "We have always believed that the best orthopaedic products are developed by working closely with surgeons. Exeter's 50 years of success are a testament to that philosophy, and I look forward to seeing how Exeter and Mako continue to advance the field of joint replacement and transform patient lives for the next 50 years."
Since its launch, the Exeter hip stem has been implanted globally in over 2 million patients, with 1 million happening in the last 10 years alone.6 “The Exeter hip is one of the great success stories of the expertise of university researchers and healthcare experts combining for the meaningful benefit of patients,” said Janice Kay, University of Exeter Provost. “It’s a collaboration at the heart of our ongoing world-leading research on a range of orthopaedic and musculoskeletal treatments. We’re so proud of the global legacy of Exeter hip. As research continues to emerge to demonstrate its endurance, I trust the Exeter hip will continue to be celebrated for years.”
Key Exeter milestones
1970 - Original Exeter introduced into clinical practice
1972 - Acetabular pressurisation technique launched
1976 - Matt Exeter stem introduced
1980 - Femoral pressurisation and canal plug technique launched
1986 - Reintroduction of the polished stainless-steel Exeter
1987 - First femoral impaction grafting case performed
1988 - Exeter universal taper stem introduced
1997 - Asia-Pacific stem size range launched – 30mm, 33mm and 35.5mm offsets
1998 - X-change III Impaction grafting instruments launched
1998 - Long revision Exeter stems (200mm, 220mm, 240mm and 260mm) launched
2000 - Sucker Aspirator instrument launched to aid acetabular pressurization
2000 - Contemporary Flanged cup launched
2001 - V40 Exeter Stem introduced with addition of 44mm/0 50mm/1 and 50mm/2 stem sizes
2006 - Short revision Exeter stem (cement-in-cement technique) launched
2007 - Long revision fully tapered 205mm 37.5mm offset Exeter stem launched
2009 - 56mm offset stem introduced
2010 - X3 RimFit cemented cup launched
2011 - 1 million Exeter stems implanted
2014 - Exeter 125mm short stems launched
2015 - New Modular and DA Specialty Instruments launched
2016 - First Mako SmartRoboticsTM case performed with Exeter hip stem
2020 - Exeter celebrates 50 years and 2 million Exeter stems implanted
References
1. Ling RS, Charity J, Lee AJ, Whitehouse SL, Timperley AJ, Gie GA. The long-term results of the original Exeter polished cemented femoral component: a follow-up report. J Arthroplasty. 2009;24(4):511-517. doi:10.1016/j.arth.2009.02.002
2. Petheram TG, Whitehouse SL, Kazi HA, et al. The Exeter Universal cemented femoral stem at 20 to 25 years: A report of 382 hips. Bone Joint J. 2016;98-B(11):1441-1449. doi:10.1302/0301-620X.98B11.37668
3. Keeling P, Howell JR, Kassam AM, et al. Long-term survival of the cemented Exeter Universal stem in patients 50 years and younger: an update on 130 hips. J Arthroplasty. 2020;35(4):1042-1047. doi:10.1016/j.arth.2019.11.009
4. Evans JT, Blom AW, Timperley AJ, et al. Factors associated with implant survival following total hip replacement surgery: A registry study of data from the National Joint Registry of England, Wales, Northern Ireland and the Isle of Man. PLoS Med. 2020;17(8):e1003291. Published 2020 Aug 31. doi:10.1371/journal.pmed.1003291
5. Ling RSM, Lee AJC, Gie GA, et al., eds. The Exeter Hip: 40 Years of Innovation in Total Hip Arthroplasty. Exeter Hip Publishing; 2010:25-37.
6. Based on Stryker sales data 1970-2020
A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery.
The information presented is intended to demonstrate the breadth of Stryker's product offerings. A surgeon must always refer to the package insert, product label and/or instructions for use before using any of Stryker's products. The products depicted are CE marked according to the Medical Device Regulation 2017/745 or the Medical Device Directive 93/42/EEC. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your sales representative if you have questions about the availability of products in your area.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: Exeter, Mako, RimFit, SmartRobotics, Stryker, V40, X3, X-Change. All other trademarks are trademarks of their respective owners or holders.
EXETER-COM-4_27545
