SpineJack® system controlled anatomical restoration

According to the AO principles of fracture management, the fracture of a weight-bearing joint is first reduced then stabilized using fixation.1 The concept of the SpineJack® system is to allow controlled anatomical restoration in order to facilitate early mobilization and weight-bearing.
Anatomical restoration consists of achieving sagittal and coronal balance. These are key factors for kyphosis management and reduction of adjacent level fractures.2-7
Vertebral endplate restoration has been described as having a positive influence on disc creeping, disc degeneration, compensatory curvatures or facet joint arthritis
Studies have shown a correlation between vertebral deformation and clinical problems such as post-traumatic kyphosis, which has been depicted as a serious post-traumatic deformity.3,13
EU indication:
The SpineJack® system is indicated for use in the reduction of mobile spinal fractures that may result from osteoporosis, trauma (fractures types A according to the Magerl classification), and malignant lesions (myeloma or osteolytic metastasis).
The SpineJack® system is intended to be used in combination with validated bone cement, and to be placed, using a transpedicular approach, through a vertebra pedicle with a minimum internal diameter (see the Specifications section of the IFU), as verified with a preoperative CT scan.
US indication:
The SpineJack® Expansion Kit is indicated for use in the reduction of painful osteoporotic vertebral compression fractures and traumatic vertebral compression fractures (type A fractures according to the AO/Magerl classification) with or without posterior instrumental fixation. It is intended to be used in combination with Stryker VertaPlex® radiopaque bone cement and VertaPlex® HV radiopaque bone cement.
Refer to IFU for complete instructions and warnings and precautions applicable for devices.
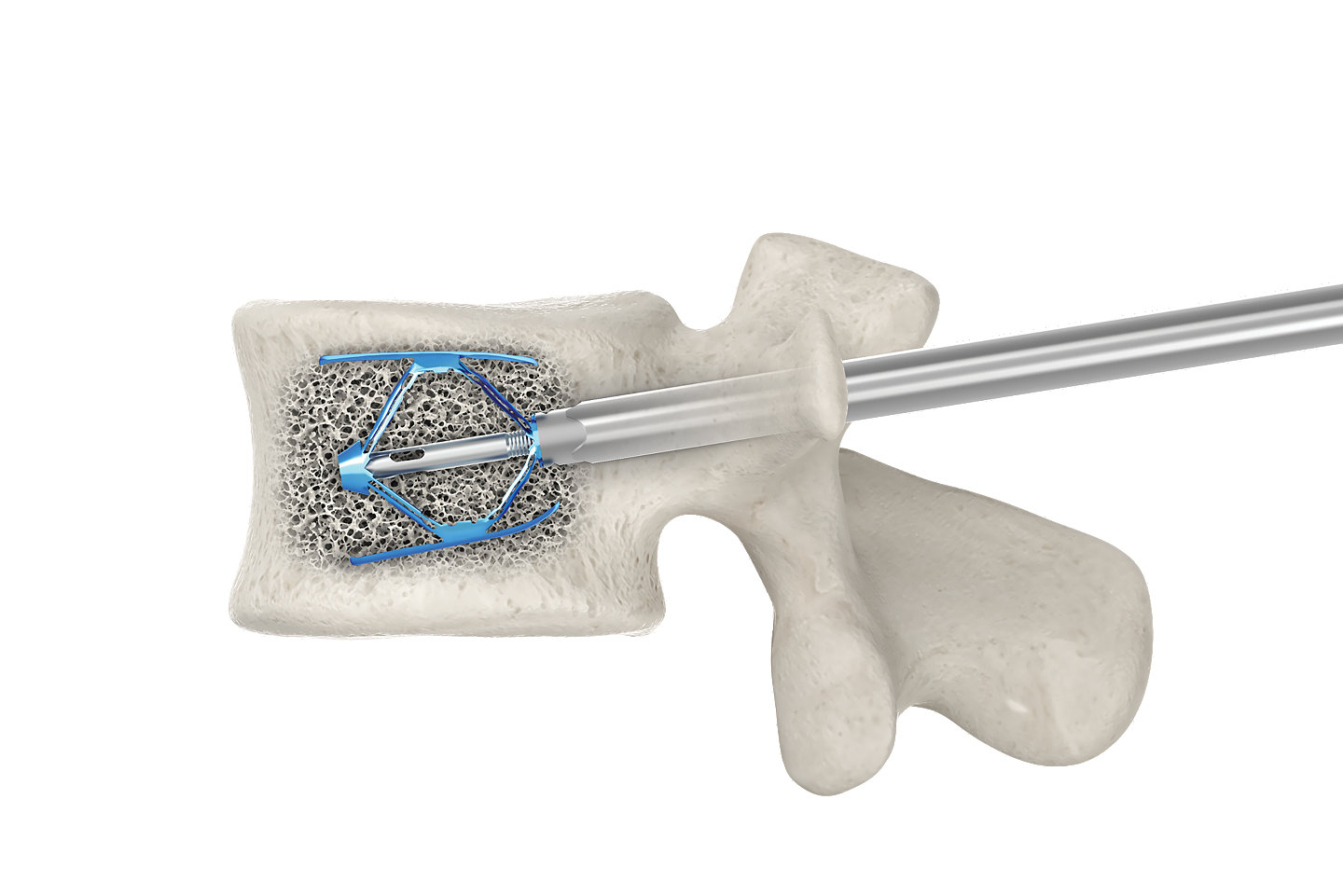

Implant positioning
Controlled by specific instrumentation
Implant positioning in both sagittal and transverse planes can help achieve the appropriate fit for the fracture’s shape and the patient’s anatomy.

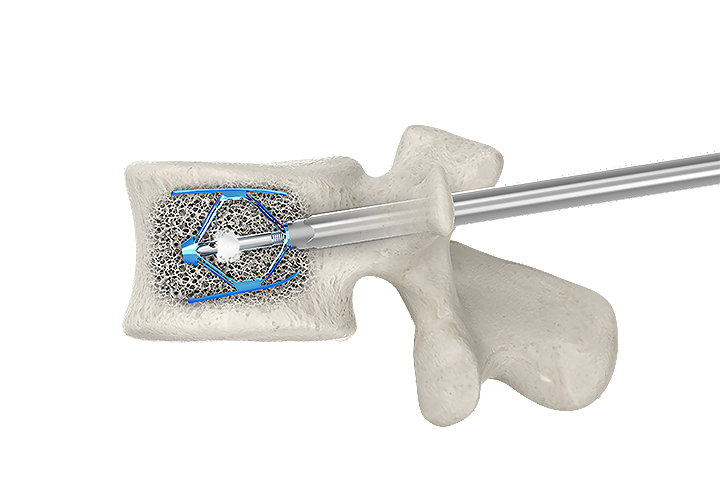
Implant expansion
Controlled by millimetric implants expansion
Millimetric expansion of the implant can be maintained until the bone cement is injected.
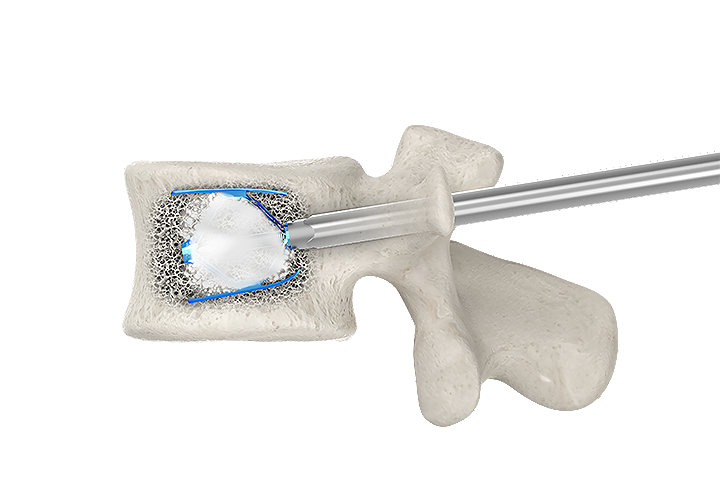
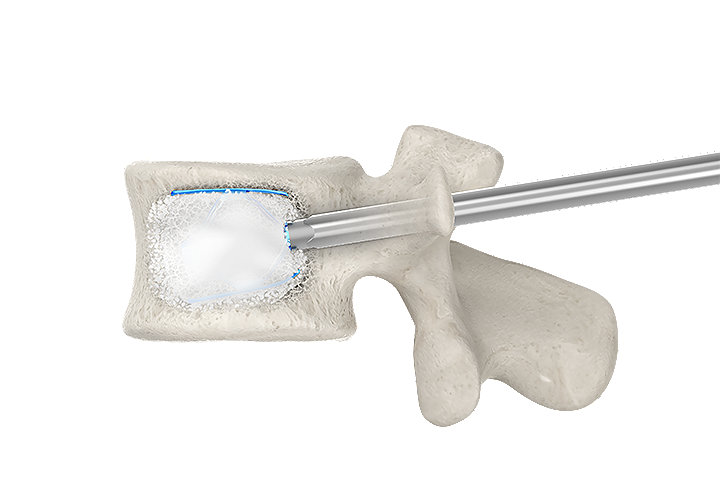
PMMA cement positioning and interdigitation
Controlled by PMMA cement fixed pathway
Fixed pathway for the insertion of PMMA cement through the implant helps minimize the possibility of posterior cement spread and risk of leakage into the spinal canal.14
By optimizing PMMA cement positioning and interdigitation, the SpineJack® system provides controlled craniocaudal expansion for anatomical restoration to allow for better functional recovery of the injured disc.15-17
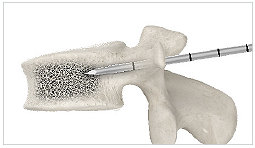
1. 11G access cannula
Insert the 11G access cannula through the pedicle into the posterior one-third of the vertebral body.
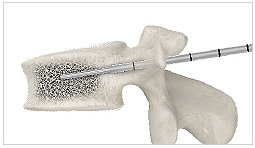
2. Guidewire
Insert the guidewire through the 11G access cannula halfway into the vertebral body, then, remove the tube portion of the 11G access cannula.
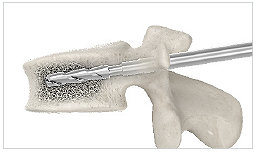
3. Reamer
Drill into the posterior one-third of the vertebral body. Remove the guidewire. Continue to drill until the desired position of the implant is reached.
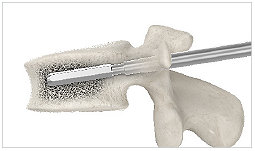
4. Template
Clean the implant site with the template.
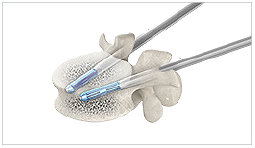
5. Unexpanded
Two SpineJack® implants are placed into the vertebral body.
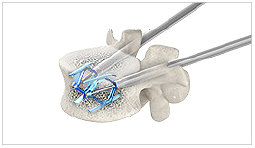
6. SpineJack® expanded (no cement)
The SpineJack® implants are expanded to help reducing the fracture and restore the anatomy.
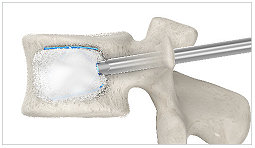
7. SpineJack® expanded (with cement)
Cement is injected to stabilize the fracture.
References
- Buckley, R., Moran, C., & Apivatthakakul, T. (2017). AO principles of fracture management. Davos Platz, CH: AO Foundation.
- Oner F et al. Changes in the disc space after fractures of the thoracolumbar spine. Journal of Bone and Joint Surgery. British volume. 1998; 80(5):833-839.
- Oda I et al. Does spinal kyphotic deformity influence the biomechanical characteristics of the adjacent motion segments: An in vivo animal model. Spine. 1999; 24(20):2139-2146.
- Schlaich C et al. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporosis International. 1998; 8(3):261-267.
- Lombardi I et al. Evaluation of pulmonary function and quality of life in women with osteoporosis. Osteoporosis International. 2005; 16(10):1247-1253.
- Yang H et al. Changes of pulmonary function for patients with osteoporotic vertebral compression fractures after kyphoplasty. Journal of Spinal Disorders and Techniques. 2007; 20(3):221-225.
- Wang X et al. Kyphosis recurrence after posterior short-segment fixation in thoracolumbar burst fractures. Journal of Neurosurgery: Spine. 2008; 8(3):246-254.
- Tzermiadianos M et al. Altered disc pressure profile after an osteoporotic vertebral fracture is a risk factor for adjacent vertebral body fracture. European Spine Journal. 2008; 17:1522-1530.
- Kerttula L et al. Post-traumatic findings of the spine after earlier vertebral fracture in young patients: clinical and MRI study. Spine. 2000; 25(9):1104-1108.
- Cinotti G et al. Degenerative changes of porcine intervertebral disc induced by vertebral endplate injuries. Spine. 2005; 30(2):174-180.
- Brinckmann P et al. The influence of vertebral body fracture, intradiscal injection, and partial discectomy on the radial bulge and height of human lumbar discs. Spine. 1985; 10(2):138-145.
- Malcolm B et al. Post-traumatic kyphosis. A review of forty-eight surgically treated patients. Journal of Bone and Joint Surgery. 1981; 63(6):891-899.
- Whitesides T. Traumatic kyphosis of the thoracolumbar spine. Clinical Orthopaedics and Related Research. 1977; 128:78-92.
- Jacobson R et al. Re-expansion of osteoporotic compression fractures using bilateral SpineJack implants: Early clinical experience and biomechanical considerations. Cureus. 2019; 11(4):e4572.
- Noriega D et al. Long-term benefits of percutaneous anatomical restoration of vertebral compression fractures linked to malignancy. Turkish Neurosurgery. 2016; 26(4):608-614.
- Noriega D et al. Clinical performance and safety of 108 SpineJack implantations: 1-year results of a prospective multicentre single-arm registry study. BioMed Research International. 2015; 2015:173872.
- Vanni et al. Third-generation percutaneous vertebral augmentation systems. Journal of Spine Surgery. 2016; 2(1):13-20.
Disclaimer
Bone cement: serious adverse events, some with fatal outcome, associated with the use of bone cements for vertebroplasty, kyphoplasty and sacroplasty include myocardial infarction, cardiac arrest, cerebrovascular accident, pulmonary embolism and cardiac embolism. Although it is rare, some adverse events have been known to occur beyond a year or more postoperatively. Additional risks exist with the use of bone cement. Please see the IFU for a complete list of potential risks.
2020-27613
