We use cookies to customize content for your viewing and for analytics. If you continue to browse this website, we will assume that you are happy to receive all our cookies. For further information please read our cookie policy.
Surpass Evolve™
Flow Diverter
Flow diversion simplified
Stryker's flow diverter system combining years of flow diversion research, extensive physician feedback and Stryker engineering prowess, to develop a highly optimized 64-wire flow diverter.

Lower push force
The flexibility profile of the solid core wire is designed to match anatomy and partner with Excelsior XT-27 Microcatheter Standard Straight, for lower friction forces, even in highly tortuous vessels.
Delivery wire stiffness designed to match anatomy

Testing performed by Stryker. Data on file at Stryker. Bench test results may not necessarily be indicative of clinical performance.
Reliable opening
Engineered to maintain the radial pressure of Surpass Streamline Flow Diverter for reliable implant opening, distal to proximal.
Testing performed by Stryker. Data on file at Stryker. Bench test results may not necessarily be indicative of clinical performance.
64-wire mesh density
Surpass Evolve is the only 64-wire cobalt chromium flow diverter* that maintains consistent mesh density across diverse neurovascular anatomy.

Uniform wall apposition
A 64-wire implant was chosen to enhance implant opening, conformability and vessel wall apposition.
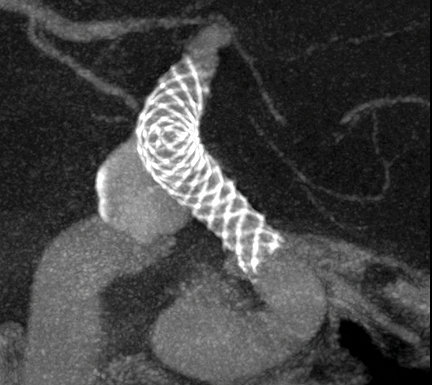
Image courtesy of Dr. Vitor Mendes Pereira, Toronto Western Hospital.
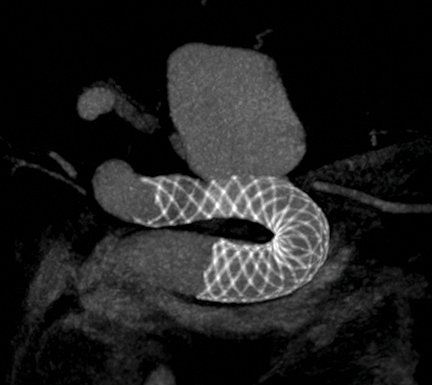
Image courtesy of Dr. Timo Krings, Toronto Western Hospital.
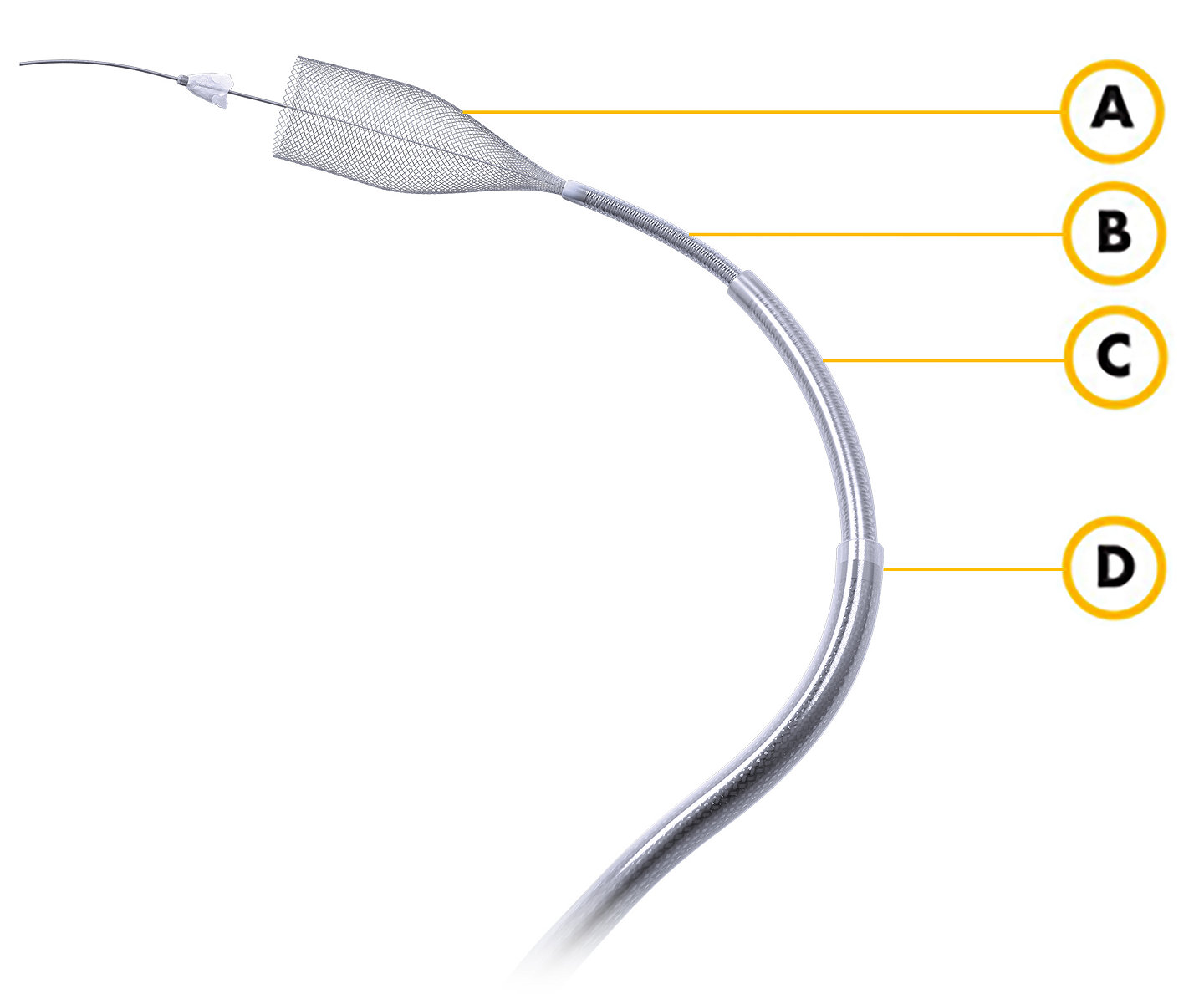
A) Surpass Evolve
Flow Diverter
B) Excelsior XT-27
Microcatheter
C) AXS Catalyst 5
Distal Access Catheter
D) AXS Infinity LS and Plus
Long Sheath
Designed for ease of use and optimized flow diversion, the Surpass Evolve Flow Diverter has demonstrated safety and effectiveness in the treatment of unruptured intracranial aneurysms around the world.
*As of February 2025
This information is intended solely for the use of healthcare professionals.
A physician must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that physicians be trained in the use of any particular product before using it in a procedure. The information presented is intended to demonstrate the breadth of Stryker product offerings. A physician must always refer to the package insert, product label and/or instructions for use before using any Stryker product. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Stryker representative if you have questions about the availability of Stryker products in your area.
Stryker or its affiliated entities own, use, or have applied for the following trademarks or service marks: AXS Catalyst, AXS Infinity, Excelisor, Surpass Evolve, Stryker. All other trademarks are trademarks of their respective owners or holders.
The absence of a product, feature, or service name, or logo from this list does not constitute a waiver of Stryker’s trademark or other intellectual property rights concerning that name or logo.
AP004770 v1.0
