HeartSine samaritan PAD 350/360P
HeartSine samaritan PAD 350/360P
Make sure help is always within reach.
The compact and easy-to-use HeartSine AEDs empower first responders with lifesaving technology, while integrated Wi-Fi connectivity ensures the devices are ready when needed most.
Readiness made easy.
The first person to respond to a sudden cardiac arrest patient might be a coworker, a caregiver, or even a bystander. Are you ensuring they have immediate access to the technology needed to help save a life?

Product highlights
HeartSine samaritan PAD 350P and 360P AEDsThe semi-automatic HeartSine samaritan PAD 350P AED and fully automatic HeartSine samaritan PAD 360P AED, available with or without Wi-Fi connectivity, offer value and environmental protection in one of the smallest and lightest packages available.

Portable, lightweight and rugged
Smaller and lighter than other defibrillators at just 1.08 kgs, with up to a 56% smaller footprint to accommodate limited space in an office, plane or vehicle.
An IP56 rating offers the industry's highest level of protection against dust and water.

Clinically validated technology
Proprietary electrode technology and SCOPE biphasic technology automatically adjust for differences in patient response to intervention.
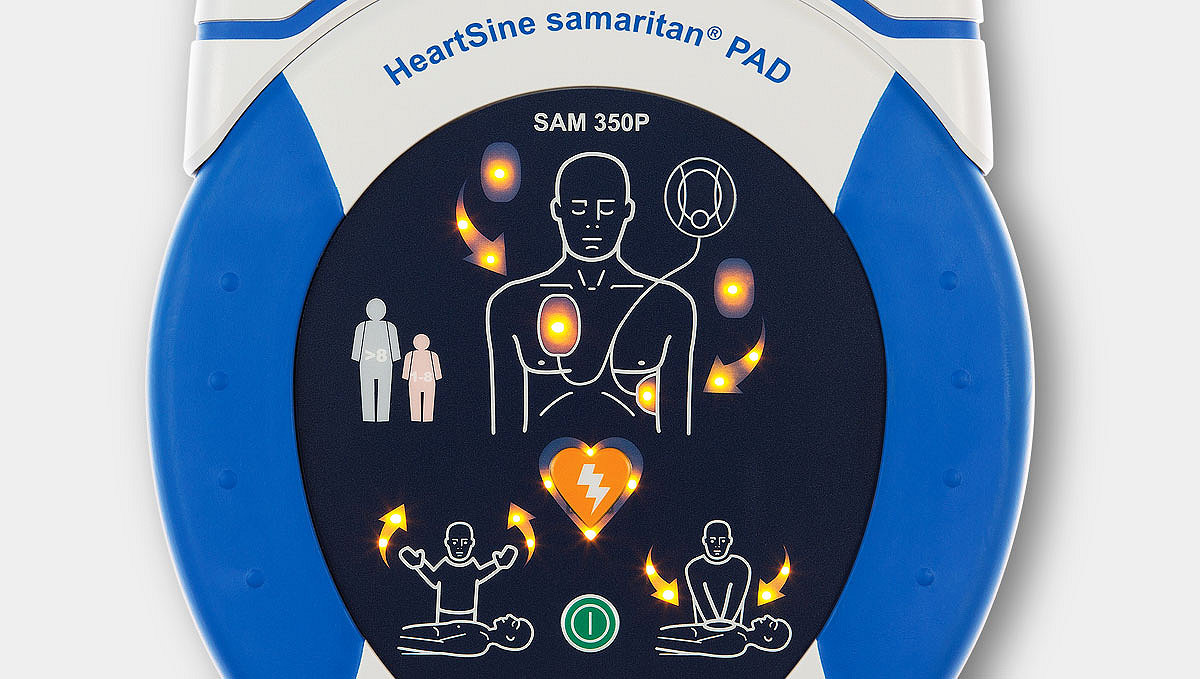
Simple, user-friendly operation
Easy-to-understand visual and voice prompts guide the rescuer through the entire resuscitation process, including CPR—a key link in the chain of survival.
Simple, straightforward operation with just an on/off button (and a shock button on the HeartSine samaritan PAD 350P).

Self-monitoring AED program
Battery not charged? Electrodes out of date? The LIFELINKcentral AED program manager monitors each HeartSine AED connected to a Wi-Fi network and alerts you to anything that may affect device readiness—all automatically.
This greatly reduces the effort and expense of managing your AED program, while increasing your program’s readiness and effectiveness.
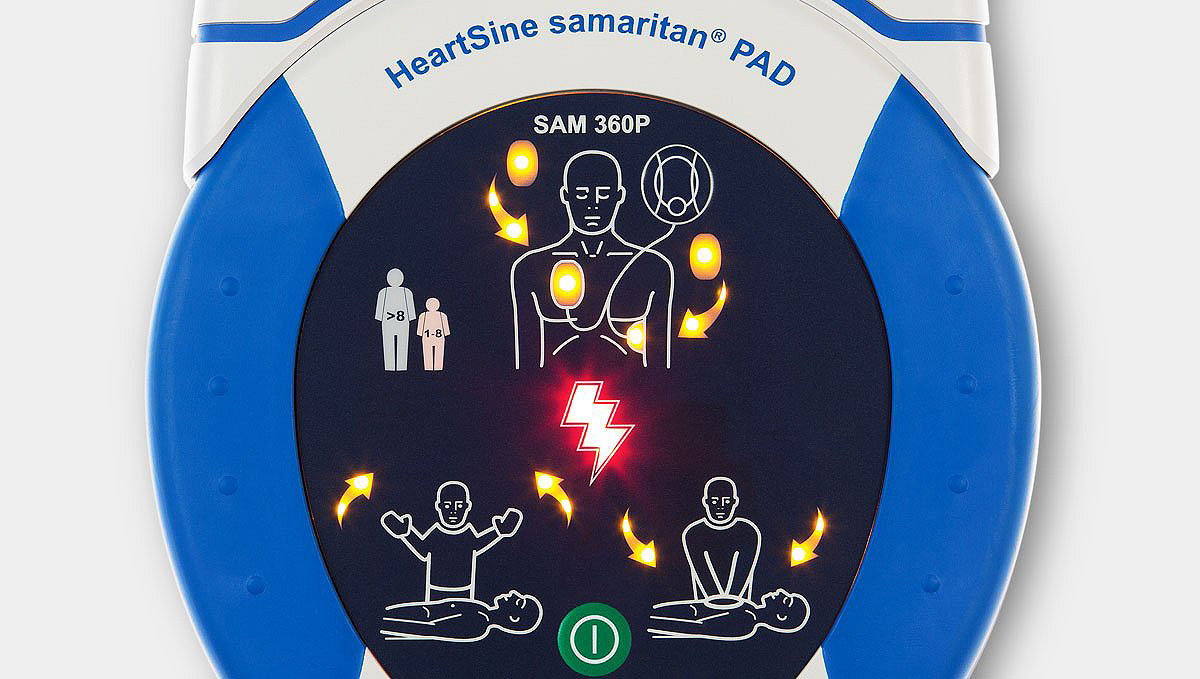
Automatic shock delivery
(HeartSine samaritan PAD 360P)
Analyzes heart rhythm and automatically delivers a shock (if needed), eliminating the need for the rescuer to push a shock button.
Service and support
Low cost of ownership
HeartSine defibrillators come with the practical Pad-Pak – an integrated battery and electrode single-use cartridge with one expiration date, which simplifies AED maintenance. With a shelf life of four years from date of manufacture, the Pad-Pak offers practical savings over other defibrillators that require separate battery and electrode replacements.
Train and learn
Empower a team of confident responders. The easy-to-use HeartSine Trainer guides users through simulated analysis, energy delivery and prompted CPR with the look and feel of a live HeartSine device – without the actual charge and discharge of an electrical shock.
Program Management
Management solutions tailored to your needs
Our AED program management options are supported by trained AED experts who are just a quick phone call or email away. We offer complete solutions from start-up to ongoing management, with the flexibility to customize for your environment and resources.
Take the next step
Want to talk to your Sales Representative? Please complete the form below and we’ll connect you with your local representative.
Related products
This document is intended solely for the use of healthcare professionals. A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that healthcare professionals be trained in the use of any particular product before using it.
The information presented is intended to demonstrate Stryker’s product offerings. A healthcare professional must always refer to operating instructions for complete directions for use indications, contraindications, warnings, cautions, and potential adverse events, before using any of Stryker’s products. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your representative if you have questions about the availability of Stryker’s products in your area. Specifications subject to change without notice. The products depicted are CE marked in accordance with applicable EU Regulations and Directives.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: LIFEPAK, LIFENET, LUCAS, HealthEMS, RevNet, CODE-STAT, Physio-Control, HeartSine, samaritan, LIFEPAK CR, TrueCPR, REDI-CHARGE, QUIK-COMBO, EDGE, cprINSIGHT, ClearVoice, QUIK-STEP, LIFEPAK TOUGH, HomeSolutions.net, RELI, REDI-PAK, Shock Advisory System, LIFELINKcentral. All other trademarks are trademarks of their respective owners or holder. The yellow and black color scheme is a registered trademark of Stryker Corporation.
LIFEPAK devices: CE Class IIb (0123)
HeartSine device: CE Class IIb (0123)
LUCAS device: CE Class IIb (2460)
EC REP: Stryker European Operations Limited | Anngrove, IDA Business & Technology Park | Carrigtwohill, Co. Cork, T45 HX08 | Ireland
3346809_A
