We use cookies to customise content for your viewing and for analytics. If you continue to browse this website, we will assume that you are happy to receive all our cookies. For further information, please read our cookie policy.
A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient and must refer to the package insert, product label and/or instructions for use before using any Stryker product.
Shown to improve CPR quality, on the move and over long durations5-7
The LUCAS device extends the reach of care by maintaining chest compressions during transport to advanced lifesaving therapies, including ECMO (extracorporeal membrane oxygenation) or PCI (percutaneous coronary interventions) in the cath lab. By increasing provider safety8,11,12, avoiding fatigue over long durations9 and reducing transport risks by allowing caregivers to sit belted2,10, the LUCAS device can help calm the scene and provide an extra pair of hands.
Key benefits
Help keep your team safe
Help reduce caregiver risk during patient transport and in the cath lab with reduced x-ray exposure8,11-12,20 and less body strain to the CPR provider3,19.
Bridge to definitive care
Deliver guidelines-consistent chest compressions for as long as needed* to allow difficult-to-resuscitate patients access to advanced lifesaving therapies. The LUCAS device helps bring life-saving interventions like ECMO/ECPR within reach of patients who don’t respond to traditional resuscitation methods21-22.
* When using multiple batteries or an external power source, battery typically lasts for 45 minutes of operation.17
Improve CPR quality
The LUCAS device provides high quality guidelines-consistent chest compressions, shown by research to increase the chances of good patient outcomes, while helping to prevent caregiver fatique, variation in CPR quality and CPR related injuries in healthcare providers.
The LUCAS device has demonstrated increased blood flow to the brain13-14 and achieved higher EtCO2 values compared to manual compressions.15-16
Enhance team efficiency
Calm chaotic scenes and enable caregivers to focus their skills and judgement where it matters most. Relying on LUCAS to provide guidelines-consistent high-quality CPR allows you to focus on what’s important: life-saving interventions, speedy diagnosis and treatment of underlying conditions.
See LUCAS in action
"If someone had told me about an 8-hour cardiac arrest. I wouldn't have believed it. But this truly happened."
– Alessandro Forti, MD
HEMS Head Coordinator, Italy
Product highlights
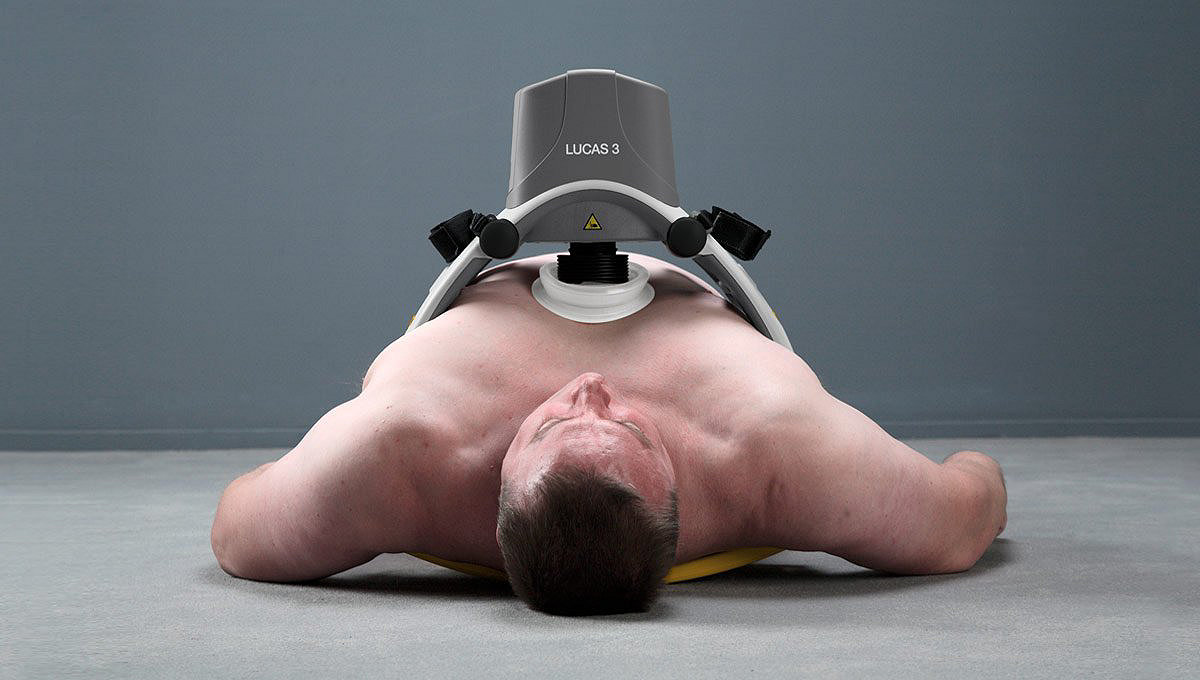
Easy to learn and use23
- Low-profile back plate for patient application
- Spacious support structure accommodates larger patients17
- A median interruption time of 7 seconds demonstrated when transitioning from manual to mechanical CPR during clinical use18
- Straps secure patient arms and device during transport
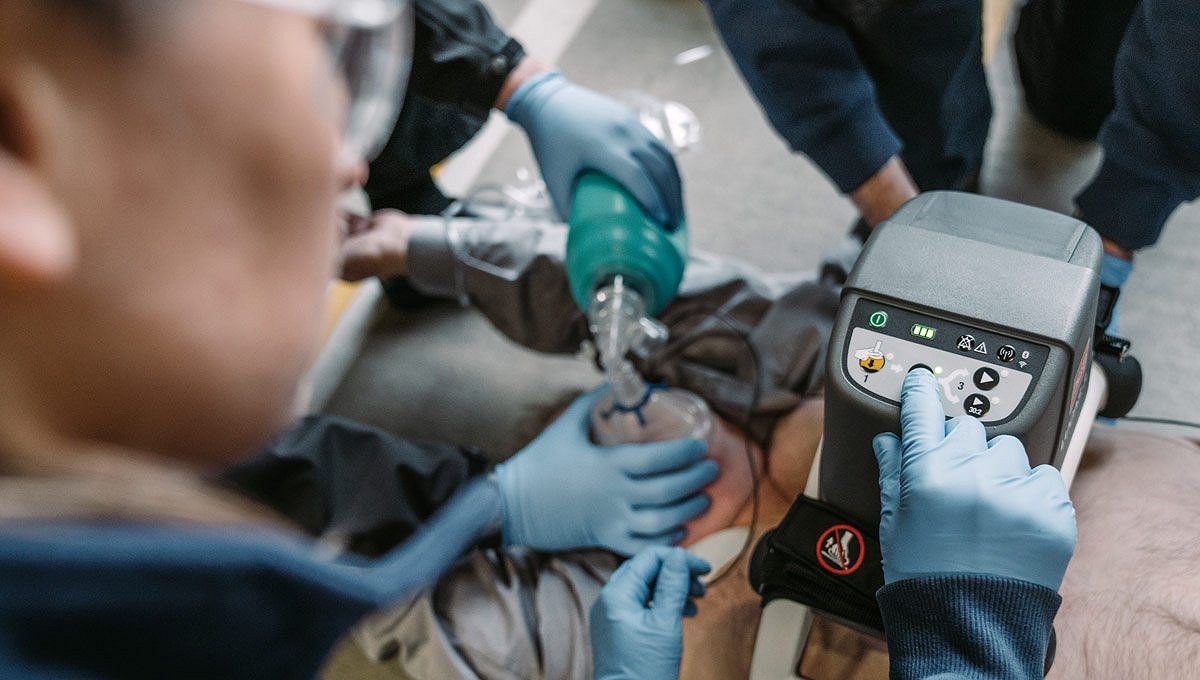
Configure to your protocols*
- Configure compression rate, depth, ratio and alerts to your protocols via wireless LIFENET connectivity
- Adjustable ventilation alerts, pause length and count
- Timer to remind rhythm and pulse checks
- Set auto-lowering and pressure pad parameters to your preferences

Access and share post-event data*
- Make QI/QA documentation faster and easier with emailed post-event reports
- Connect wirelessly via Bluetooth and WiFi to the LIFENET System
- Easy-access LUCAS device data enables productive post-event review
- Integrate with CODE-STAT (version 11 and higher) data review software

Convenient to store and carry
- Battery operation encourages portability
- Lightweight and compact carrying case includes a window for quick battery checks
- External power supply enables prolonged operation and charging
- Device can be charged inside the case through access port

Facilitate asset management*
- Confirm device or fleet status via LIFENET connectivity
- Receive battery age notifications
*LUCAS 3, v3.1, LIFENET and CODE-STAT are available in major markets. Please contact your local Stryker representative for more information about the LUCAS device and data connectivity.
LUCAS web training
Read, listen and watch how to apply the LUCAS chest compression system for training and re-training purposes.
Users are encouraged to do refresher trainings at least once a year. Please select your LUCAS device below.
Interested in learning more? Connect with an expert.
Manufacturer
Jolife AB, a part of Stryker
Scheelevägen 17
Ideon Science Park
SE-223 70 Lund, Sweden
Tel: +46 (0) 46 286 50 00
info@lucas-cpr.com
Technical support
We'll work with you to quickly assess your situation and find the best solution.
Product resources
Find product resources such as brochure, case studies, one-sheets and more.
Product documentation
Find Instructions for Use (IFUs) and Declaration of Conformity (DoC) for the LUCAS device.
1. Beesems SG, Hardig BM, Nilsson A, Koster RW, Force and depth of mechanical chest compressions and their relation to chest height and gender in an out-of-hospital setting, Resuscitation, 2015;91:67-72.
2. Becker L, Zaloshnja E, Levick N, et al. Relative risk of injury and death in ambulances and other emergency vehicles. Accident analysis and prevention. 2003;35(6): 941-948.
3. Jones A, Lee R. Cardiopulmonary resuscitation and back injury in ambulance officers. International Archives of Occupational and Environmental Health.2005 May;78 (4); 332-336.
4. Edelson, et al. Interim guidance for basic and advanced life support in adults, children, and neonates with suspected or confirmed COVID-19. Circulation. 2020.
5. Putzer G, Braun P, Zimmerman A, et al. LUCAS compared to manual cardiopulmonary resuscitation is more effective during helicopter rescue–a prospective, randomized, cross-over manikin study. Am J Emerg Med.2013 Feb;31(2):384-9.
6. Gyory R, Buchle S, Rodgers D, et al. The efficacy of LUCAS in prehospital cardiac arrest scenarios: A crossover mannequin study. West J Emerg Med.2017;18(3):437-45.
7. Olasveengen TM, Wik L, Steen PA. Quality of cardiopulmonary resuscitation before and during transport in out-of-hospital cardiac arrest. Resuscitation. 2008;76(2):185-90.
8. AHA Guidelines: Panchal A, Bartos J, Cabanas J, et al. Part 3: Adult Basic and Advanced Life Support. 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2), S366–S468.
9. Sugerman NT, Edelson DP, Leary M, et al. Rescuer fatigue during actual inhospital cardiopulmonary resuscitation with audiovisual feedback: a prospective multicenter study. Resuscitation 2009;80:981-4.
10. Fox J, Fiechter R, Gerstl P, et al. Mechanical versus manual chest compression CPR under ground ambulance transport conditions. Acute Cardiac Care 2013;15:1-6.
11. ERC European Resuscitation Council Guidelines for Resuscitation 2015, Resuscitation. 2015;95:1-311.
12. Soar J, Böttiger BW, Carli P, et al., European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation. Vol 161 (2021) p115-151
13. Carmona Jiménez F, Padró PP, García AS, et al. Cerebral flow improvement during CPR with LUCAS, measured by Doppler. Resuscitation. 2011, 82S1:30, AP090. [Also published in a longer version, in Spanish with English abstract, in Emergencias. 2012;24:47-49].
14. Rubertsson S, Karlsten R. Increased cortical cerebral blood flow with LUCAS; a new device for mechanical chest compressions compared to standard external compressions during experimental cardiopulmonary resuscitation. Resuscitation 2005:65(3);357-363.
15. Axelsson C, Karlsson T, Axelsson A, et al. Mechanical active compression-decompression cardiopulmonary resuscitation (ACD-CPR) versus manual CPR according to pressure of end tidal carbon dioxide (PETCO2) during CPR in out-of-hospital cardiac arrest (OHCA). Resuscitation. 2009, 80(10):1099-1103.
16. Chandler P, Ibrahim M. AS099. Manual chest compressions versus LUCAS 2© – A comparative study of End-tidal carbon dioxide levels during in-hospital resuscitation. Resuscitation. 2017;118(suppl 1):e41. Oral presentation
17. Data on file, Stryker LUCAS 3 chest compression system Instructions for Use 101034-01.
18. Levy M, Yost D, Walker R, et al., A quality improvement initiative to optimize use of a mechanical chest compression device within a high-performance CPR approach to out-of-hospital cardiac arrest resuscitation. Resuscitation. 2015;92:32-37
19. Jones A. Can cardiopulmonary resuscitation injure the back? Resuscitation, 2004 Apr; 61(1):63-7
20. William P, Rao P, Kanakadandi U, et al. Mechanical cardiopulmonary resuscitation in and on the way to the cardiac catheterization laboratory. Circ J. 2016:25;80(6):1292-1299.
21. Forti A, Brugnaro P, Rauch S, et al. Hypothermic Cardiac Arrest With Full Neurologic Recovery After Approximately Nine Hours of Cardiopulmonary Resuscitation: Management and Possible Complications. Ann Emerg Med. 2019;73(1):52-57.
22. Bonnemeier H, Simonis G, Olivecrona G, et al. Continuous mechanical chest compression during in-hospital cardiopulmonary resuscitation of patients with pulseless electrical activity. Resuscitation. 2011;82:155-9
23. Pocock H, Deakin C, Quinn T, et al. Human factors in pre-hospital research: lessons from the PARAMEDIC trial. Emerg Med J Online. Feb 25, 2016
EC-LUC-SYK-1570550_REV-0_en_gb