We use cookies to customise content for your viewing and for analytics. If you continue to browse this website, we will assume that you are happy to receive all our cookies. For further information, please read our cookie policy.
Acute Care product disclaimer statement
This document is intended solely for the use of healthcare professionals.
A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that healthcare professionals be trained in the use of any particular product before using it.
The information presented is intended to demonstrate the breadth of Stryker product offerings. A healthcare professional must always refer to the package insert, product label and/or instructions for use before using any Stryker product.
Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Stryker representative if you have questions about the availability of Stryker products in your area.
Stryker Corporation or its divisions or other corporate-affiliated entities own, use or have applied for the following trademark(s) or service mark(s): Rapr. Round Wraps, MV3, ProCuity, SV2, ST1 Series, Prime Series Stretchers, IsoTour, TruRize, Eye Surgery Stretcher, Fluoroscopy Stretcher, Gynnie, Prime TC, Prime X, Stretcher Chair, Transport Stretcher, ComfortGel, ComfortGel SE, Eole DC, IsoFlex SE, Prime Big Wheel, Prime Fifth Wheel, Prime Zoom, ProForm and Ultra Comfort SE. All other trademarks are trademarks of their respective owners or holders.
This material is not intended for distribution outside the EU and EFTA.
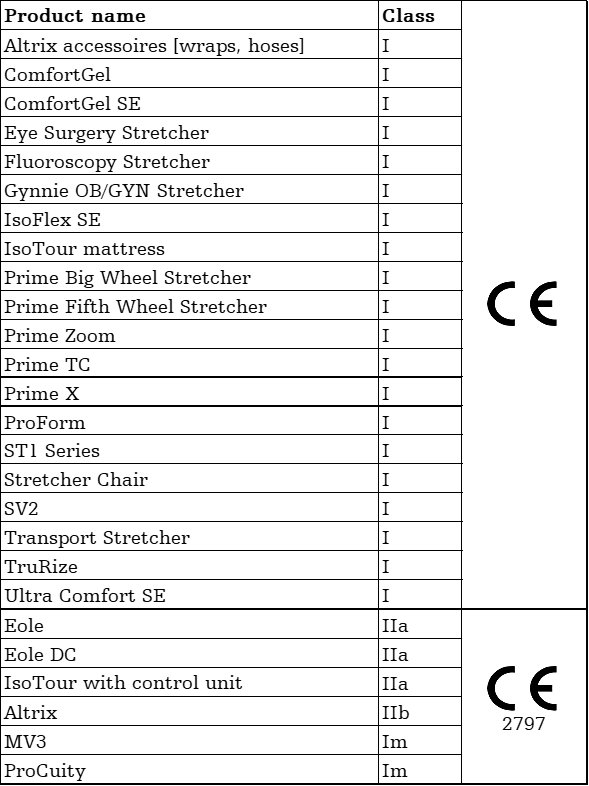
The products depicted are CE marked in accordance with applicable EU Regulations and Directives.

Stryker Medical
3800 E. Centre Ave.
Portage, MI 49002 USA
