Target Tetra® Detachable Coil
A big deal
for small spaces.
For over 10 years, we have been continuously making Target Coils better.
With Target Tetra, we’ve combined a stable, tetrahedral shape with
incredible softness, making it an excellent option for treating small spaces
from frame to finish.
Target Tetra is our softest coil, yet has enhanced shape stability, so you’re
never compromising one advantage for the other.

The Target family has a proven legacy. And now, with Target Tetra, we are reshaping the possibilities.
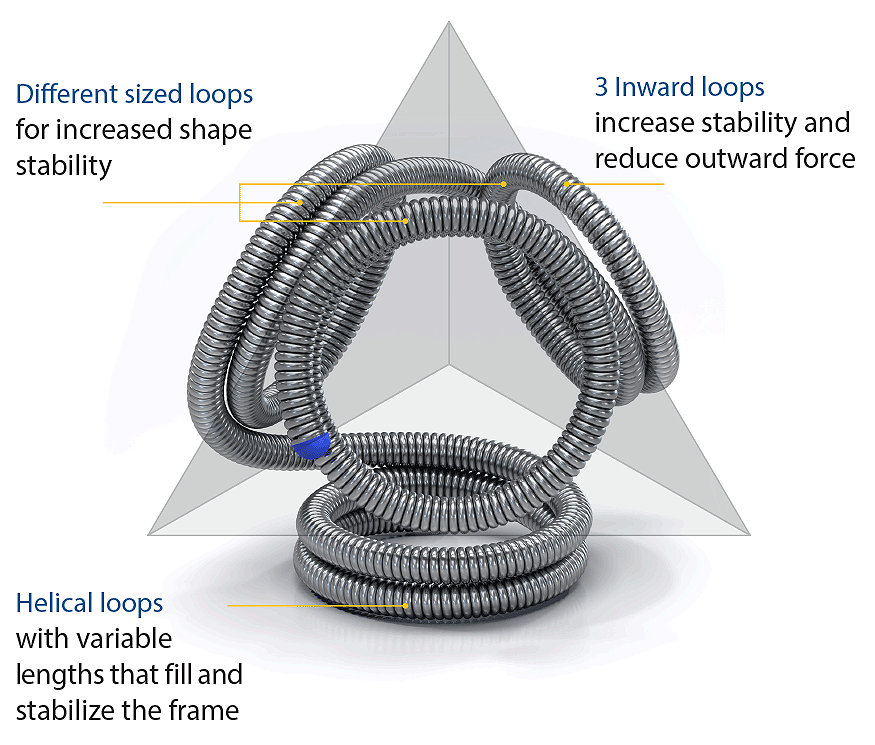
The unique tetrahedral shape is designed to:
1 Treat small aneurysms from frame to finish
2 Increase stability
3 Reduce coil herniation
Our softest coil was designed for small spaces. Whether you’re treating a small aneurysm or using alongside other Target Detachable Coils for filling and finishing, Target Tetra has you covered.
Click here to see the Target Tetra® Detachable Coil in action.

Target Tetra is 28% softer than Target Nano and 67% softer than Target Ultra.
Coil softness is especially important when working in small spaces because it helps reduce aneurysm rupture risk.
Bench testing is not necessarily indicative of clinical performance. Data on file.
Trusted hemorrhagic solution
Target Tetra Detachable Coils are compatible with:
1 Excelsior SL-10 Microcatheter, Excelsior XT-17 Microcatheter, or Excelsior 1018 Microcatheter
2 AXS Catalyst 5 Distal Access Catheter
3 AXS Infinity LS Long Sheath
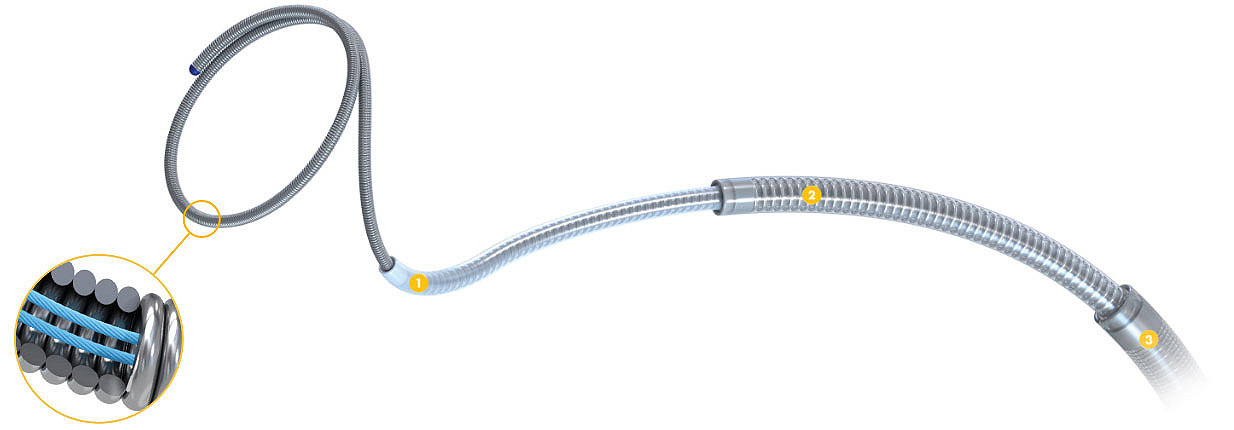
Its braided stretch resistant suture is designed to allow for a smoother delivery and reduced microcatheter kickback.
The same smooth and stable delivery system as legacy Target Coils
Aneurysm size: H: 2.72mm, W: 2.95mm, N: 2.08mm
Products used: Target Tetra Detachable Coil 3mm x 6cm,
Excelsior SL-10 Straight Microcatheter
Video taken by Stryker.
Ruptured basilar fenestration aneurysm
Physician: Dr. Warren Kim, San Francisco, CA
Aneurysm dimensions:
Height: 1.5mm
Width: 1.9mm
Neck: 1.3mm
Coils used:
Target Tetra 1.5mm x 3cm
“Microcatheter stability was great and crucial for this case. It would have been an extremely difficult case without Tetra.”
– Dr. Warren Kim
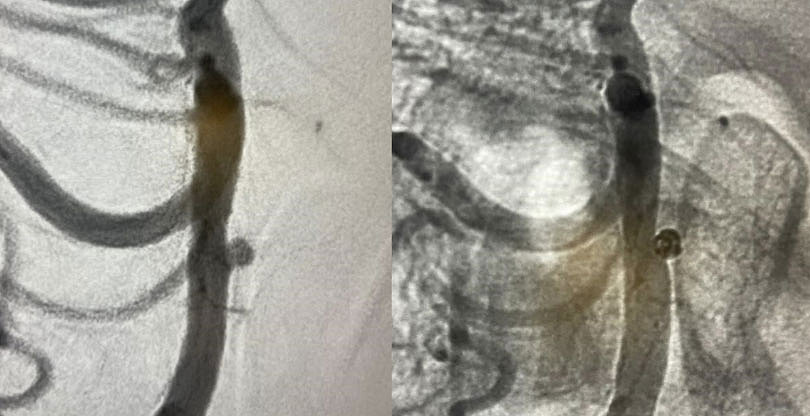
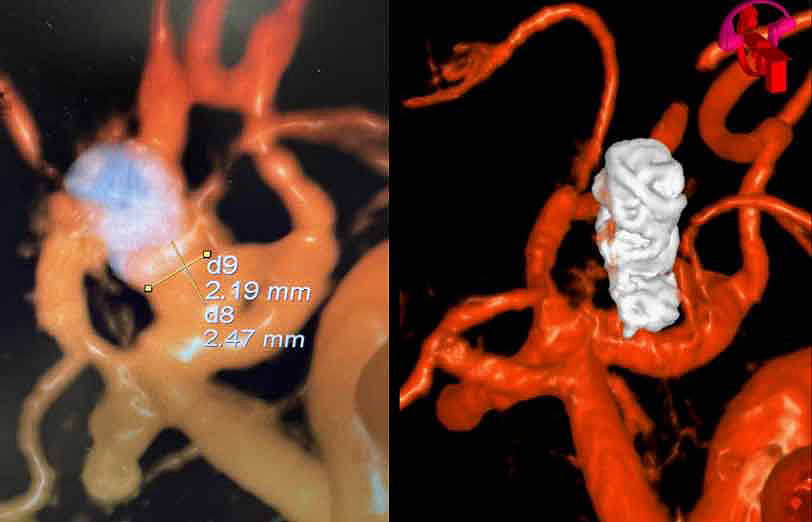
Residual ACOM aneurysm
Physician: Dr. Rahul B. Jadhav, Little Rock, AK
Aneurysm dimensions:
Height: 2.47mm
Width: 2.19mm
Neck: 2mm
Coils used:
Target Tetra 3mm x 6cm, 3mm x 4.5cm, 2.5mm x 3.5cm
“In my early cases it excelled my expectations and aced the outcome.”
– Dr. Rahul B. Jadhav
Unruptured ACOM aneurysm
Physician: Dr. Satoshi Tateshima, Los Angeles, CA
Aneurysm dimensions:
Height: 4.0mm
Width: 3.5mm
Neck: 3.5mm
Coils used:
Target Tetra 3.5mm x 8cm, 2.5mm x 4.5cm, 1.5mm x 3.0cm, 1.5mm x 3.0cm, 1.5mm x 2.0cm
“It is not just a great small aneurysm framer, but also a great neck filler.”
– Dr. Satoshi Tateshima
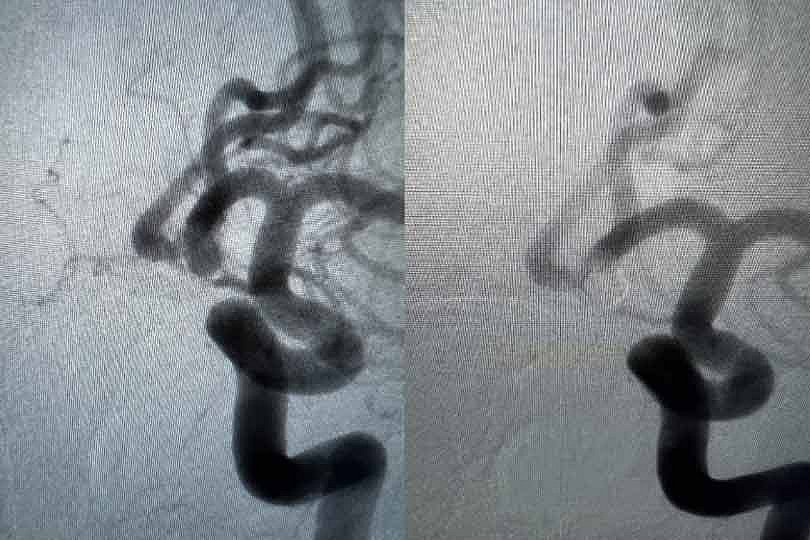
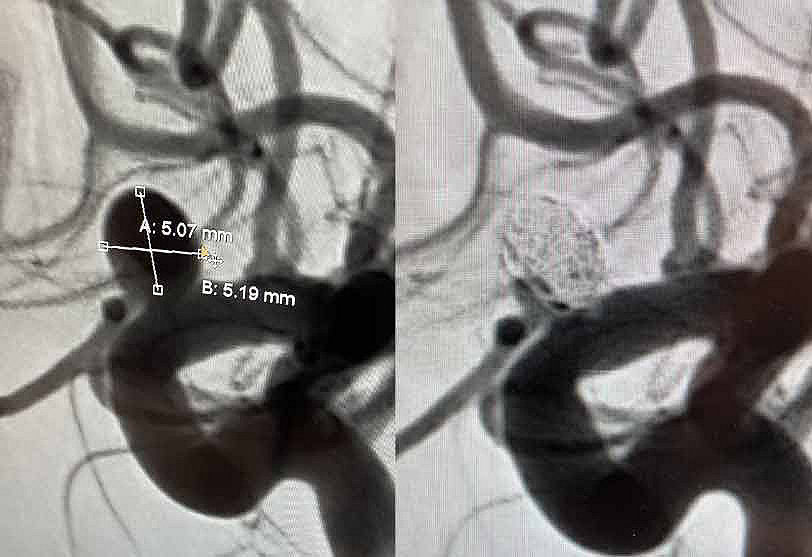
ICA (ophthalmic segment) aneurysm
Physician: Dr. Kadir Erkmen, Temple University Hospital, Philadelphia, PA
Aneurysm dimensions:
Height: 5.19mm
Width: 5.07mm
Neck: 4.5mm
Coils used:
Target 3D 5mm x 10cm
Target Tetra 3.5mm x 8cm, 3.5mm x 8cm, 3.5mm x 6cm, 2.5mm x 4.5cm, 2.5mm x 3.5cm, 2.5mm x 3.5cm
“Tetra is the softest coil I have used and makes treatment of complex aneurysms possible, especially small ruptured aneurysms that would otherwise require neck remodeling.”
– Dr. Kadir Erkmen
Dr. Tateshima and Dr. Kim are paid consultants of Stryker. The opinions expressed by physicians are not necessarily those of Stryker. Individual experiences may vary.
| Target 360 Ultra | Target 360 Nano | Target Tetra | |
|---|---|---|---|
| SR suture | Monofilament | Monofilament | Braided multi-filament |
| Primary wire | 0.0015" | 0.00125” | 0.00125” |
| Primary coil OD | 0.010” | 0.010” | 0.010” |
| Secondary OD | 2-5mm | 1-3.5mm | 1.5-3.5mm |
| Lengths | 3-15cm | 2-6cm | 2-10cm |
| Detachment | Electrolytic | Electrolytic | Electrolytic |
| MC compatibility | Excelsior SL-10 Microcatheter, Excelsior XT-17 Microcatheter, Excelsior 1018 Microcatheter |
Excelsior SL-10 Microcatheter, Excelsior XT-17 Microcatheter, Excelsior 1018 Microcatheter |
Excelsior SL-10 Microcatheter, |
Ask your sales rep about Target Tetra sizing considerations.
For over 10 years, we have been continuously making our Target Coils better. Now we’re introducing Target Tetra, which brings a new shape and softness into one coil.
Ultra registry takeaways
The ULTRA Registry proved that it is safe to treat small aneurysms, now with Target Tetra, you can feel even more confident when treating small aneurysms from frame to finish.
Ultra registry
The ULTRA Registry is an investigator-initiated prospective, multicenter U.S. registry of patients with small (≤5mm) intracranial aneurysms treated exclusively with Target Ultra and Target Nano coils.
Primary endpoints: Target aneurysm occlusion and recurrence rates.
Secondary endpoints: Device and/or procedure related adverse events, aneurysm hemorrhage intraoperatively or during follow-up.
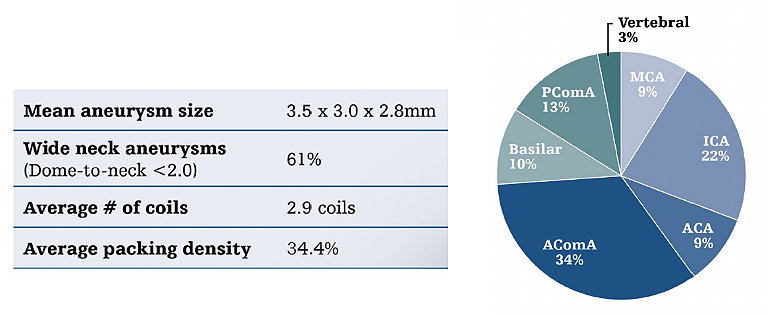
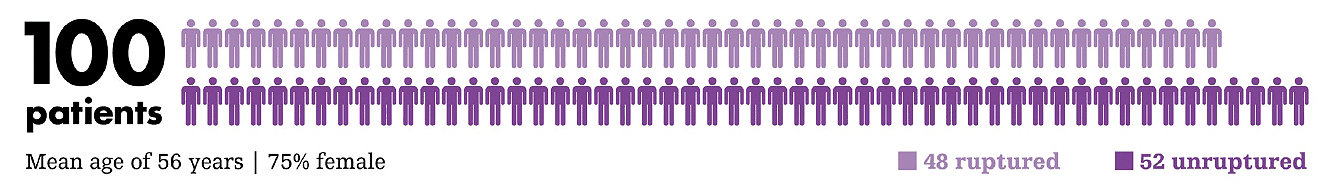
Key findings
Core lab adjudicated results confirm the excellent safety and efficacy profiles of Target Ultra and Nano coils in the treatment of ruptured and unruptured small intracranial aneurysms.

Disclaimer: This Investigator Initiated Study was funded wholly or in part by Stryker. The funding source was not involved in study design, monitoring, data collection, statistical analyses, interpretation of results, or manuscript writing.
Stryker or its affiliated entities own, use, or have applied for the following trademarks or service marks:1018, AXS Catalyst, Excelsior, Nano, SL-10, Stryker, Target, Target Tetra, Target XL, AXS Infinity LS. All other trademarks are trademarks of their respective owners or holders.
The absence of a product, feature, or service name, or logo from this list does not constitute a waiver of Stryker’s trademark or other intellectual property rights concerning that name or logo.
This document is intended solely for the use of healthcare professionals.
A physician must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that physicians be trained in the use of any particular product before using it in a procedure. The information presented is intended to demonstrate the breadth of Stryker product offerings. A physician must always refer to the package insert, product label and/or instructions for use before using any Stryker product. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Stryker representative if you have questions about the availability of Stryker products in your area.
Copyright © 2024
AP004152 v4.0
